


 الفيزياء الكلاسيكية
الفيزياء الكلاسيكية
 الكهربائية والمغناطيسية
الكهربائية والمغناطيسية
 علم البصريات
علم البصريات
 الفيزياء الحديثة
الفيزياء الحديثة
 النظرية النسبية
النظرية النسبية
 الفيزياء النووية
الفيزياء النووية
 فيزياء الحالة الصلبة
فيزياء الحالة الصلبة
 الليزر
الليزر
 علم الفلك
علم الفلك
 المجموعة الشمسية
المجموعة الشمسية
 الطاقة البديلة
الطاقة البديلة
 الفيزياء والعلوم الأخرى
الفيزياء والعلوم الأخرى
 مواضيع عامة في الفيزياء
مواضيع عامة في الفيزياء|
Read More
Date: 11-12-2015
Date: 9-11-2015
Date: 15-10-2015
|
Sadi Carnot

Reflections
The story of thermodynamics begins in 1824 in Paris. France had been rocked to its foundations by thirty-five years of war, revolution, and dictatorship. A king had been executed, constitutions had been written, Napoleon had come and gone twice, and the monarchy had been restored twice. Napoleon had successfully marched his armies through the countries of Europe and then disastrously into Russia. France had been invaded and occupied and had paid a large war indemnity.
In 1824, a technical memoir was published by a young military engineer who had been born into this world of social, military, and political turmoil. The engineer’s name was Sadi Carnot, and his book had the title Reflections on the Motive Power of Fire. By “motive power” he meant work, or the rate of doing work, and “fire” was his term for heat. His goal was to solve a problem that had hardly even been imagined by his predecessors. He hoped to discover the general operating principles of steam engines and other heat engine devices that supply work output from heat input. He did not quite realize his purpose, and his work was largely ignored at the time it was published, but after Carnot’s work was rediscovered more than twenty years later it became the main inspiration for subsequent work in thermodynamics.
Lazare Carnot
Although he always worked on the fringes of the scientific world of his time, Sadi Carnot did not otherwise live in obscurity. His father, Lazare, was one of the most powerful men in France during the late eighteenth and early nineteenth centuries. Sadi was born in 1796 in the Paris Luxembourg Palace when Lazare was a member of the five-man executive Directory. Lazare Carnot served in high level positions for only about four years, but his political accomplishments and longevity were extraordinary for those turbulent times. Before joining the government of the Directory, he was an influential member of the all-powerful Committee of Public Safety led by Maximilien de Robespierre. In that capacity, Lazare was responsible for the revolutionary war efforts. His brilliant handling of logistics and strategy salvaged what might otherwise have been a military disaster; in French history textbooks he is known as “the great Carnot” and “the organizer of victory.” He was the only member of the Committee of Public Safety to survive the fall of Robespierre in 1794 and to join the Directory. A leftist coup in 1797 forced him into exile, but he returned as Napoleon’s war minister. (He had given Napoleon command of the Italian army in 1797.) Napoleon’s dictatorial ways soon became evident, however, and Lazare, unshakable in his republican beliefs, resigned after a few months. But he returned once more in 1814, near the end of the Napoleonic regime, first as the governor of Antwerp and then as Napoleon’s last minister of the interior.
Lazare Carnot’s status in history may be unique. Not only was he renowned for his practice of politics and warfare; he also made important discoveries in science and engineering. A memoir published in 1783 was, according to Lazare’s biographer, Charles Gillispie, the first attempt to deal in a theoretical way with the subject of engineering mechanics. Lazare’s goal in this and in later work in engineering science was to abstract general operating principles from the mechanical workings of complicated machinery. His aim, writes Gillispie, “was to specify in a completely general way the optimal conditions for the operation of machines of every sort.” Instead of probing the many detailed elements of machinery design, as was customary at the time, he searched for theoretical methods whose principles had no need for the details.
Lazare Carnot’s main conclusion, which Gillispie calls the “principle of continuity of power,” asserts that accelerations and shocks in the moving parts of machinery are to be avoided because they lead to losses of the “moment of activity” or work output. The ideal machine is one in which power is transmitted continuously, in very small steps. Applied to water machines (for instance, waterwheels), Lazare’s theorem prescribes that for maximum efficiency there must be no turbulent or percussive impact between the water and the machine, and the water leaving the machine should not have appreciable velocity.
Lazare’s several memoirs are not recognized today as major contributions to engineering science, but in an important sense his work survives. His approach gave his son Sadi a clear indication of where to begin his own attack on the theory of heat engines. Lazare’s views on the design of water engines seem to have been particularly influential. Waterwheels and other kinds of hydraulic machinery are driven by falling water, and the greater the fall, the greater the machine’s work output per unit of water input. Sadi Carnot’s thinking was guided by an analogy between falling water in water engines and falling heat in heat engines: he reasoned that a heat engine could not operate unless its design included a high-temperature body and a low-temperature body between which heat dropped while it drove the working parts of the machine.
Heat Engines, Then and Now
The heat engines of interest to Sadi Carnot were steam engines applied to such tasks as driving machinery, ships, and conveyors. The steam engine invented by a Cornishman, Arthur Woolf, was particularly admired in France in the 1810s and 1820s. Operation of the Woolf engine is diagrammed in figure 1.1. Heat Q2
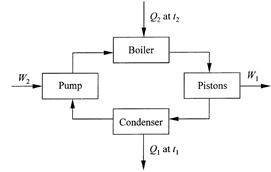
Figure 1.1. Diagram of the Woolf steam engine.
was supplied at a high temperature t2 by burning a fuel, and this heat generated steam at a high pressure in a boiler. The steam drove two pistons and they provided the work output W1. (In this chapter and elsewhere in this part of the book, keep in mind that the symbol t represents temperature and not time, as in chapters 1 and 2.) The steam leaves the pistons at a decreased pressure and temperature. Heat Q1 was then extracted in a condenser where the steam was further cooled to a still lower temperature t1 and condensed to liquid water. Finally, the liquid water passed through a pump, which restored the high pressure by expending work W2, and low-temperature, pressurized water was returned to the boiler. This is a cycle of operations, and its net effect is the dropping of heat from the high temperature t2 to the low temperature t1, with work output W1 from the pistons and a much smaller work input W2 to the pump.
The Woolf steam engine and its variations have evolved into a vast modern technology. Most contemporary power plants operate similarly. The scale is much larger in the modern plants, the operating steam pressures and temperatures are higher, and the working device is a turbine rather than pistons. But the concept of heat falling between a high and a low temperature with network output again applies.
Carnot’s Cycle
Sadi Carnot had the same ambitions as his father. He hoped to abstract, from the detailed complexities of real machinery, general principles that dictated the best possible performance. Lazare’s analysis had centered on ideal mechanical operation; Sadi aimed for the mechanical ideal, and also for ideal thermal operation.
He could see, first of all, that when heat was dropped from a high temperature to a low temperature in a heat engine it could accomplish something. His conceptual model was based on an analogy between heat engines and water engines. He concluded that for maximum efficiency a steam engine had to be designed so it operated with no direct fall of heat from hot to cold, just as the ideal water engine could not have part of the water stream spilling over and falling directly rather than driving the waterwheel. This meant that in the perfect heat engine, hot and cold parts in contact could differ only slightly in temperature. One can say, to elaborate somewhat, that the thermal driving forces (that is, temperature differences) in Carnot’s ideal heat engine have to be made very small. This design had more than an accidental resemblance to Lazare Carnot’s principle of continuity in the transmission of mechanical power.
To make it more specific, Carnot imagined that his ideal heat engine used a gaseous working substance put through cyclic changes something like the steam in the pistons of the Woolf steam engine. Carnot’s cycles consisted of four stages:
1. An isothermal (constant-temperature) expansion in which the gas absorbed heat from a heat “reservoir” kept at a high temperature t2.
2. An adiabatic (insulated) expansion that lowered the temperature of the gas from t2 to t1.
3. An isothermal compression in which the gas discarded heat to a reservoir kept at the low temperature t1.
4. An adiabatic compression that brought the gas back to the original high temperature t2.
Stages 1 and 3 accomplish the heat fall by absorbing heat at a high temperature and discarding it at a low temperature. More work is done by the gas in the expansion of stage 1 than on the gas in the compression of stage 3; and amounts of work done on and by the gas in stages 2 and 4 nearly cancel each other. Thus, for each turn of the cycle, heat is dropped from a high temperature to a low temperature, and there is network output.
Carnot’s Principle
To summarize, Carnot constructed his ideal heat engine, as Lazare had made his ideal machinery, so that all its parts and stages functioned continuously in very small steps under very small thermal and mechanical driving forces. This and the necessity for operating in cycles between two fixed temperatures were, Carnot realized, the main features required for all ideal heat engine operation. The special features of the four-stage gas cycle were convenient but unnecessary; other ways could be found to drop the heat between the two heat reservoirs and produce work output.
Carnot’s point of view insists that the forces driving an ideal heat engine be so small they can be reversed with no additional external effect and the engine made to operate in the opposite direction. Run forward, in its normal mode of operation as a heat engine, the ideal machine drops heat, let’s say between the temperatures t2 and t1, and provides work output. Run backward, with all its driving forces reversed, the ideal machine requires work input and it raises heat from t1 to t2. This is a heat pump, analogous to a mechanical device capable of pumping water from a low level to a high level. Carnot reached the fundamental conclusion that any ideal heat engine, operated as it had to be by very small driving forces, was literally “reversible.” All of its stages could be turned around and, with no significant effect in the surroundings, the heat engine made into a heat pump, or vice versa. This reversibility aspect of ideal heat engine operation led Carnot to his main result, a proof that any ideal heat engine operating between heat reservoirs maintained at t2 and t1, had to supply the same work output W for a given heat input Q2. If two ideal heat engines had different work outputs W and W' with W' larger than W, say, the engine with higher work output W' could be used to drive the
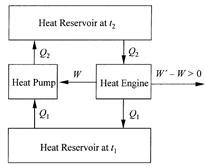
Figure 1.2. Illustration of impossible perpetual work output obtained by linking two ideal heat engines with different work outputs, W and W'.
engine with lower work output W in reverse to pump the heat Q2 back to its original thermal level in the upper heat reservoir, and with network output W'- W (fig. 1.2).
If this composite device had been possible, it would have served as a perpetual-motion machine because it supplied work output with no need to replenish the heat supply in the upper heat reservoir; every unit of heat dropped through the heat engine was restored to the upper reservoir by the heat pump. In other words, this composite heat engine could have worked endlessly without having to burn fuel. Lazare Carnot had relied heavily on the axiom that perpetual motion of any kind was physically impossible, and this was another one of the father’s lessons learned by the son. Sadi Carnot also categorically rejected the possibility of perpetual motion and therefore concluded that the two ideal heat engines in the composite machine had to have the same work output, that is, W -W'.
Put more formally, Carnot’s conclusion was that all ideal heat engines operating in cycles between the two temperatures t1 and t2 with the heat input Q2 have the same work output W. Design details make no difference. The working material can be steam, air, or even a liquid or solid; the working part of the cycle can be a gas expansion, as in Carnot’s cycle, or it can be something else. The work output W of the ideal heat engine is precisely determined by just three things, the heat input Q2 and the temperatures t1 and t2 of the two reservoirs between which the heat engine operates. This statement expresses “Carnot’s principle.” It was an indispensable source of inspiration for all of Carnot’s successors.
Carnot’s Function
To continue with his analysis, Carnot had to deduce what he could concerning the physical and mathematical nature of ideal engine operation. Here he seems to have exploited further his idea that heat engines do work by dropping heat from a higher to a lower temperature. It seemed that the ability of heat to do work in a heat engine depended on its thermal level expressed by the temperature t, just as the ability of water to do work in a water engine depends on its gravitational level.
Carnot emphasized a function F(t) that expressed the ideal heat engine’s operating efficiency at the temperature t. He made three remarkable calculations of numerical values for his function F(t). These calculations were based on three different heat engine designs that used air, boiling water, and boiling alcohol as the working materials. Carnot’s theory required that ideal heat engine behavior be entirely independent of the nature of the working material and other special design features: values obtained for F(t) in the three cases had to be dependent only on the temperature t. Although the primitive data available to Carnot for the calculation limited the accuracy, his results for F(t) seemed to satisfy this requirement. No doubt this success helped convince Carnot that his heat engine theory was fundamentally correct.
To complete his theory, Carnot had to find not just numbers but a mathematical expression for his function F(t). In this effort, he was unsuccessful; he could see only that F(t) decreased with increasing temperature. Many of Carnot’s successors also became fascinated with this problem. Although in the end Carnot’s function was found to be nothing more complicated than the reciprocal of the temperature expressed on an absolute scale, it took no fewer than eight thermodynamicists, spanning two generations, to establish this conclusion unequivocally; five of them (Carnot, Clausius, Joule, Helmholtz, and Thomson) were major figures in nineteenth-century physics.
Publication and Neglect
Sadi Carnot’s work was presented as a privately published memoir in 1824, one year after Lazare Carnot’s death, and it met a strange fate. The memoir was published by a leading scientific publisher, favorably reviewed, mentioned in an important journal and then for more than twenty years all but forgotten. With one fortunate exception, none of France’s esteemed company of engineers and physicists paid any further attention to Carnot’s memoir.
One can only speculate concerning the reasons for this neglect. Perhaps Carnot’s immediate audience did not appreciate his scientific writing style. Like his father, whose scientific work was also ignored at first, Carnot wrote in a semi popular style. He rarely used mathematical equations, and these were usually relegated to footnotes; most of his arguments were stated verbally. Evidently Carnot, like his father, was writing for engineers, but his book was still too theoretical for the steam-engine engineers who should have read it. Others of the scientific establishment, looking for the analytical mathematical language commonly used at the time in treatises on mechanics, probably could not take seriously this unknown youth who insisted on using verbal science to formulate his arguments. It didn’t help either that Carnot was personally reserved and wary of publicity of any kind. One of his rules of conduct was, “Say little about what you know and nothing at all about what you don’t know.” In the end, like Newton with the Principia, Carnot missed his audience.
In time, Carnot probably would have seen his work recognized, if not in France, perhaps elsewhere where theoretical research on heat and heat engines was more active. But Carnot never had the opportunity to wait for the scientific] world to catch up. In 1831, he contracted scarlet fever, which developed into “brain fever.” He partially recovered and went to the country for convalescence. But later, in 1832, while studying the effects of a cholera epidemic, he became a cholera victim himself. The disease killed him in hours; he was thirty-six years old. Most of his papers and other effects were destroyed at the time of his death, the customary precaution following a cholera casualty.
After Carnot
The man who rescued Carnot’s work from what certainly would otherwise have been oblivion was Emile Clapeyron, a former classmate of Carnot’s at the Ecole Polytechnique. It was Clapeyron who, in a paper published in the Journal de l’Ecole Polytechnique in 1834, put Carnot’s message in the acceptable language of mathematical analysis. Most important, Clapeyron translated into differential equations Carnot’s several verbal accounts of how to calculate his efficiency function F(t).
Clapeyron’s paper was translated into German and English, and for ten years or so it was the only link between Carnot and his followers. Carnot’s theory, in the mathematical translation provided by Clapeyron, was to become the point of departure in the 1840s and early 1850s for two second-generation thermodynamicists, a young German student at the University of Halle, Rudolf Clausius, and a recent graduate of Cambridge University, William Thomson (who became Lord Kelvin). Thomson spent several months in 1845 in the Paris laboratory of Victor Regnault. He scoured the Paris bookshops for a copy of Carnot’s memoir with no success. No one remembered either the book or its author.
In different ways, Clausius and Thomson were to extend Carnot’s work into the science of heat that Thomson eventually called thermodynamics. One of Clapeyron’s differential equations became a fixture in Thomson’s approach to thermodynamics; Thomson found a way to use the equation to define an absolute temperature scale. Later, he introduced the concept of energy, and with it resolved a basic flaw in Carnot’s theory: its apparent reliance on the caloric theory. Among Clausius’s contributions was an elaboration of Carnot’s heat engine analysis, which recognized that heat is not only dropped in the heat engine from a high temperature to a low temperature but is also partially converted to work. This was a departure from Carnot’s water engine analogy, and in later research it led to the concept of entropy.
Recognition
So, in the end, Sadi Carnot’s theory was resurrected, understood, and used. And it finally became clear that Carnot, no less than his father Lazare, should be celebrated as a great revolutionary. Born into a political revolution, Carnot started a scientific revolution. His theory was radically new and completely original. None of Carnot’s predecessors had exploited, or even hinted at, the idea that heat fall was the universal driving force of heat engines.
If Carnot’s contemporaries lacked the vision to appreciate his work, his numerous successors have, at least for posterity, repaired the damage of neglect. Science historians now regard Carnot as one of the most inventive of scientists. In his history of thermodynamics, From Watt to Clausius, Donald Cardwell assesses for us Sadi Carnot’s astonishing success in achieving Lazare Carnot’s grand goal, the abstraction of general physical principles from the complexities of machinery: “Perhaps one of the truest indicators of Carnot’s greatness is the unerring skill with which he abstracted, from the highly complicated mechanical contrivance that was the steam engine . . . the essentials, and the essentials alone, of his argument. Nothing unnecessary is included, and nothing essential is missed out. It is, in fact, very difficult to think of a more efficient piece of abstraction in the history of science since Galileo taught . . . the basis of the procedure.”
Scant records of Carnot’s life and personality remain. In the two published portraits, we see a sensitive, intelligent face, with large eyes regarding us with a steady, slightly melancholy gaze. Most of the biographical material on Carnot comes from a brief article written by Sadi’s brother Hippolyte. (Lazare Carnot was partial to exotic names for his sons.) Hippolyte’s anecdotes tell of Carnot’s independence and courage, even in childhood. As a youngster, he sometimes accompanied his father on visits to Napoleon’s residence; while Lazare and Bonaparte conducted business, Sadi was put in the care of Madame Bonaparte. On one occasion, she and other ladies were amusing themselves in a rowboat on a pond when Bonaparte appeared and splashed water on the rowers by throwing stones near the boat. Sadi, about four years old at the time, watched for a while, then indignantly confronted Bonaparte, called him “beast of a First Consul,” and demanded that he desist. Bonaparte stared in astonishment at his tiny attacker, and then roared with laughter.
The child who challenged Napoleon later entered the Ecole Polytechnique at about the same time the French military fortunes began to collapse. Two years later Napoleon was in full retreat, and France was invaded. Hippolyte relates that Sadi could not remain idle. He petitioned Napoleon for permission to form a brigade to fight in defense of Paris. The students fought bravely at Vincennes, but Paris fell to the Allied armies, and Napoleon was forced to abdicate.
Hippolyte records one more instance of his brother’s courage. Sadi was walking in Paris one day when a mounted drunken soldier galloped down the street, “brandishing his saber and striking down passers-by.” Sadi ran forward, dodged the sword and the horse, grabbed the soldier, and “laid him in the gutter.” Sadi then “continued on his way to escape from the cheers of the crowd, amazed at this daring deed.”
Sadi Carnot lived in a time of unsurpassed scientific activity, most of it centered in Paris. The list of renowned physicists, mathematicians, chemists, and engineers who worked in Paris during Carnot’s lifetime includes Pierre-Simon Laplace, Andre Marie Ampere, Augustin Fresnel, Simeon-Denis Poisson, Adrien Marie Legendre, Pierre Dulong, Alexis Petit, Evariste Galois, and Gaspard de Coriolis. Many of these names appeared on the roll of the faculty and students at the Ecole Polytechnique, where Carnot received his scientific training. Except as a student, Carnot was never part of this distinguished company. Like some other incomparable geniuses in the history of science (notably, Gibbs, Joule, and Mayer in our story), Carnot did his important work as a scientific outsider. But there is no doubt that Carnot’s name belongs on anyone’s list of great French physicists. He may have been the greatest of them all.



|
|
|
|
دراسة يابانية لتقليل مخاطر أمراض المواليد منخفضي الوزن
|
|
|
|
|
|
|
اكتشاف أكبر مرجان في العالم قبالة سواحل جزر سليمان
|
|
|
|
|
|
|
اتحاد كليات الطب الملكية البريطانية يشيد بالمستوى العلمي لطلبة جامعة العميد وبيئتها التعليمية
|
|
|