


 النبات
النبات
 الحيوان
الحيوان
 الأحياء المجهرية
الأحياء المجهرية
 علم الأمراض
علم الأمراض
 التقانة الإحيائية
التقانة الإحيائية
 التقنية الحيوية المكروبية
التقنية الحيوية المكروبية
 التقنية الحياتية النانوية
التقنية الحياتية النانوية
 علم الأجنة
علم الأجنة
 الأحياء الجزيئي
الأحياء الجزيئي
 علم وظائف الأعضاء
علم وظائف الأعضاء
 الغدد
الغدد
 المضادات الحيوية
المضادات الحيوية|
Read More
Date: 3-1-2022
Date: 21-9-2021
Date: 15-12-2021
|
Other applications of the Henderson-Hasselbalch equation
The Henderson-Hasselbalch equation can be used to calculate how the pH of a physiologic solution responds to changes in the concentration of a weak acid and/or its corresponding salt form. For example, in the bicarbonate buffer system, the Henderson-Hasselbalch equation predicts how shifts in the bicarbonate ion concentration, [HCO3−], and the carbon dioxide concentration [CO2] influence pH (Fig. 1A). The equation is also useful for calculating the abundance of ionic forms of acidic and basic drugs. For example, most drugs are either weak acids or weak bases (Fig.1B). Acidic drugs (HA) release a H+, causing a charged anion (A−) to form.
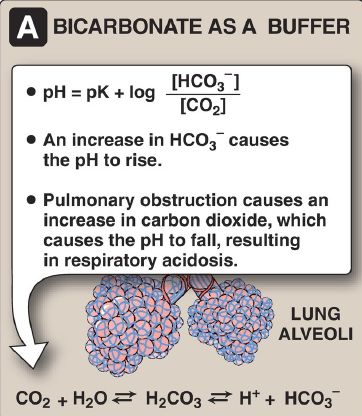
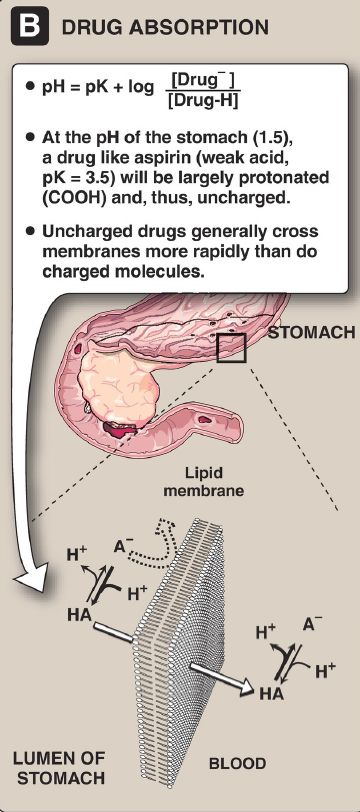
Figure 1. The Henderson-Hasselbalch equation is used to predict: (A) changes in pH as the concentrations of bicarbonate (HCO3−) or carbon dioxide (CO2) are altered and (B) the ionic forms of drugs.
Weak bases (BH+) can also release a H+. However, the protonated form of basic drugs is usually charged, and the loss of a proton produces the uncharged base (B).
A drug passes through membranes more readily if it is uncharged. Thus, for a weak acid, such as aspirin, the uncharged HA can permeate through membranes, but A− cannot. Likewise, for a weak base, such as morphine, the uncharged B form permeates through the cell membrane, but BH+ does not. Therefore, the effective concentration of the permeable form of each drug at its absorption site is determined by the relative concentrations of the charged (impermeant) and uncharged (permeant) forms. The ratio between the two forms is determined by the pH at the site of absorption and by the strength of the weak acid or base, which is represented by the pKa of the ionizable group. The Henderson-Hasselbalch equation is useful in determining how much drug is found on either side of a membrane that separates two compartments that differ in pH, for example, the stomach (pH 1.0–1.5) and blood plasma (pH 7.4).



|
|
|
|
دراسة يابانية لتقليل مخاطر أمراض المواليد منخفضي الوزن
|
|
|
|
|
|
|
اكتشاف أكبر مرجان في العالم قبالة سواحل جزر سليمان
|
|
|
|
|
|
|
اتحاد كليات الطب الملكية البريطانية يشيد بالمستوى العلمي لطلبة جامعة العميد وبيئتها التعليمية
|
|
|