


 النبات
النبات
 الحيوان
الحيوان
 الأحياء المجهرية
الأحياء المجهرية
 علم الأمراض
علم الأمراض
 التقانة الإحيائية
التقانة الإحيائية
 التقنية الحيوية المكروبية
التقنية الحيوية المكروبية
 التقنية الحياتية النانوية
التقنية الحياتية النانوية
 علم الأجنة
علم الأجنة
 الأحياء الجزيئي
الأحياء الجزيئي
 علم وظائف الأعضاء
علم وظائف الأعضاء
 الغدد
الغدد
 المضادات الحيوية
المضادات الحيوية|
Read More
Date: 6-11-2015
Date: 8-11-2015
Date: 6-11-2015
|
B Cell
Specific recognition by the immune system is mediated fundamentally by B and T lymphocytes. These cells derive from the hematopoietic stem cells through discrete differentiation pathways, although both originate in the bone marrow (and in the liver during embryonic and fetal life) . T cells mature in the thymus, whereas B cells continue to differentiate in the bone marrow itself. B cells express immunoglobulins that directly interact with native epitopes—that is, subregions of an antigen with a well-defined three-dimensional structure. Surface immunoglobulins are associated with a signaling module made of a Iga-Igb heterodimer to form the B-cell receptor (BCR). In contrast to the BCR, T-cell receptors (TCRs) do not interact directly with native epitopes, but they identify peptides derived from the original antigen as presented by molecules encoded by genes of the major histocompatibility complex (MHC).
Both the B and the T cells have to face the repertoire problem—that is, how to generate extremely large number of different immunoglobulins and TCRs to meet the requirements of recognizing an extraordinary large number of discrete epitopes. A theoretical approach suggests that this number might be as large as 1017. Because the total number of lymphocytes in a human adult averages 1012 (roughly divided into one-fifth B cells and four-fifths T cells), and taking into account that they are clonally organized, the number of BCRs and/or TCRs expressed at one given time appears much less than 1017, which implies some basic degeneracy in the immune recognition system. Nevertheless, the number of different structures must still be quite large, which therefore raises the problem of how to generate such a large repertoire with, necessarily, a limited number of genes. This is the main challenge for B cells (and T cells as well), to which the way they differentiate brings the answer.
During fetal life, B lymphocytes differentiate in the liver and then in the bone marrow, which remains active throughout life. The first steps of B-cell differentiation take place in the bone marrow, which is a primary lymphoid organ, and drives precursors derived from the hematopoietic stem cells to the immature B lymphocytes. This period of differentiation is antigen-independent and is essentially devoted to generation of the basic immunoglobulin repertoire, which is the result of a complex sequence of events that involve multiple gene rearrangements. Immature B lymphocytes migrate to secondary lymphoid organs, namely spleen, lymph nodes, tonsils, and gut-associated lymphoid tissues, including Peyer's patches. Further differentiation necessitates antigen encounter and a number of complex cellular interactions, among which cooperation between the B and T cells plays a major role. A second round of diversity is then generated, which results mostly from somatic mutations, and the final steps of differentiation, including isotype switching, lead to the emergence of plasma cells and memory B cells. Two discrete lineages of B cells have been well-characterized in the mouse and are designated B1 and B2. B1 cells express the CD5 marker, have a low level of surface IgD, and are mostly encountered in peritoneal and pleural cavities. They seem preferentially responsible for autoantibody synthesis. B2 cells represent the major and conventional B-cell population.
1.From the Hematopoietic Stem Cell to the Immature B Cell
All differentiation events that drive the emergence of the various lineages (granulocytes, erythrocytes, monocytes, megakaryocytes, lymphocytes) derive from a common hematopoietic stem cell that originates in the bone marrow. The B lineage presumably initiates from a precursor common to the B and the T lymphocytes. The main features of the molecular and cellular events that take place in the bone marrow and lead to B cell commitment are indicated in Figure 1. Three major subpopulations define the B lineage: proB, preB, and immature B cells. The successive steps of differentiation proceed from the periphery toward the center of the bone marrow and may be followed in several ways: (a) acquisition and/or loss of surface antigens (especially CD markers(identified by monoclonal antibodies, (b) identification of cytoplasmic proteins and/or messenger RNA transcripts, and (c) analysis of the gene rearrangement status for each of the three Ig gene loci: H, L, and K.
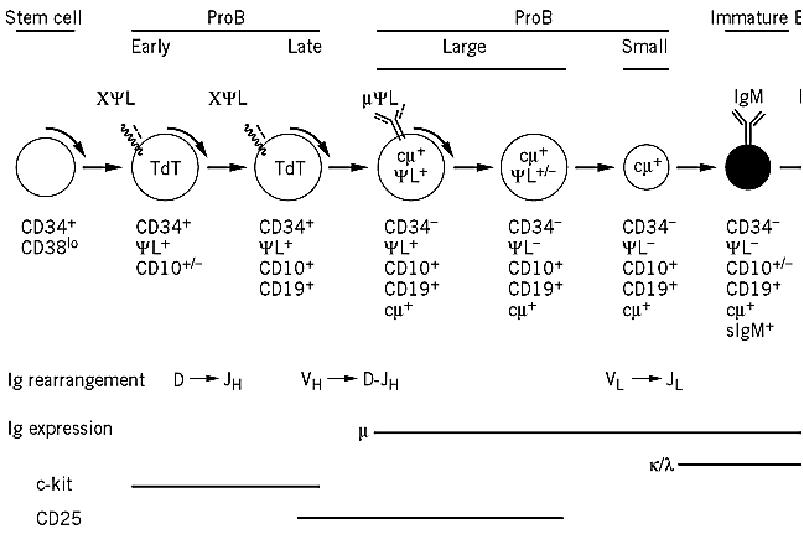
Figure 1. B-cell differentiation in humans. Early steps of B-cell differentiation take place in the bone marrow and are an The major discrete steps that lead from the hematopoietic stem cell (HSC) to the immature B lymphocyte are identified b gene rearrangements, by the presence of cytoplasmic markers, and by the expression of characteristic markers at the cell early stages that go from HSC to the early proB cell encompass several precursors that are not yet completely defined. Td deoxynucleotidyl transferase, responsible for generating N diversity; YL, surrogate light chain that combines with the Igm the preB receptor. Late events of B-cell differentiation, which take place in the periphery, are antigen-dependent. The gene organization plays a key role in mounting a T-dependent B-cell response, during which affinity is tuned upon the acquiring mutations and the biological functions of the antibodies are adapted by isotype switching. Ultimately, this differentiation cells that secrete antibodies and in memory B cells that are involved in secondary responses. Each step of differentiation by various CD surface markers, as indicated.
The first steps of differentiation involve a sequence of direct interactions of the precursors with the stromal cells, ensured by various sets of cellular adhesion molecules (CAMs). The VLA4 integrin expressed at the cell surface of the precursors interacts with stromal VCAM1, and the resulting early proB cells now express Kit, a receptor that binds the stem cell factor of stromal cells. This interaction triggers proliferation of the proB cells, which will continue to differentiate through other stimuli. Late proB cells are stimulated by a soluble growth factor also of stromal origin, interleukin-7 (IL-7), which drives proliferation of preB cells. As these steps of differentiation proceed, the cells are dividing, and Ig gene rearrangements that generate the basic repertoire take place in a sequential fashion. CD marker characterization indicates that CD34, which is already expressed in the hematopoietic stem cells, remains present at the surface of precursors and proB cells. Late proB cells can be characterized by the coexpression of CD34 with CD19, which is a very specific marker of the B lineage because it is found on all subsequent stages, with the exception of the plasma cells. CD10, also known as the common acute lymphocytic leukemia antigen (CALLA), is expressed up to the immature B-cell stage a seems expressed slightly before CD19. Other markers are expressed as differentiation proceeds, such as CD20, CD21, CD22, CD23, and CD24.
2. The Basic Ig Repertoire Develops in the Bone Marrow as B Cells Differentiate
As already stated, the main feature of B-cell differentiation is the acquisition of the basic repertoire of immunoglobulins that becomes expressed as IgM on immature B cells. IgM has the classical H2L2 organization, and both the H and L chains have variable and constant regions. The variable regions of the heavy and light chains interact in the antibody combining site, which is responsible for specific antigen recognition. Heavy and light chains are encoded by genes that are localized on three discrete gene clusters, H, k and l, located on chromosomes 14, 2, and 22, respectively. The unique feature of Ig gene organization is that they must be rearranged by the specific recombinases RAG1 and RAG2 (for recombinase activating genes) before becoming functional. The first recombination event, DH to JH, takes place in the early proB cells (see Fig. 1) and is rapidly followed by the rearrangement of one of the VH segments to D-J. Another enzyme, terminal deoxynucleotidyl-transferase, is also active in proB cells, for which it represents an additional cytoplasmic marker. This enzyme adds nucleotides in a random fashion during the joining of D to J and of V to D-J rearrangements. ProB cells that have rearranged the IgVH locus must “make” two decisions. One is to remain monoclonal with respect to H-chain production, the second is to activate rearrangement of the light chains. Both events are regulated by the m chain itself, which must be in its membrane form. Once the first allele of the IgH locus has completed the rearrangement process, the resulting gene may be functional or not, depending upon whether the recombination has generated a sequence of nucleotides with an open reading frame. Because of the triplet organization of the genetic code, this happens only once every three rearrangements. If the first rearrangement is out of frame, the second allele will recombine, with the same probability of success. A second failure will lead to cell death. Conversely, once a functional gene has been generated, the resulting heavy chain will exert a negative feedback on a further rearrangement of the IgH locus, ensuring monoclonal expression of the m chain. Much evidence suggests that the heavy chain must be expressed at the cell surface to regulate these events. Expression of the m chain at the cell surface requires that it associate with another partner, which resembles the light chain, and for this reason it is named YL or surrogate light chain. First described in the mouse and then identified in humans, the surrogate light chain is composed of two polypeptide chains, encoded by the l-like (or l5 in the mouse) and the VpreB genes, part of the regular IgL locus. The m-YL complex becomes expressed at the surface of what is now a large preB cell, which also expresses CD10 and CD19, but no longer CD34. The cell is able to undertake light-chain gene rearrangements, which occur in the order k→l. The recombination process is regulated in the same manner as that of the heavy chains, so there is only one light chain that is expressed in any given cell, either k or l (see l) Light Chains). The negative feedback on further rearrangements of the light chain genes is exerted by the complete IgM, which is now expressed at the surface of the immature B cell and has replaced the mYL complex. At this point, the Iga–Igb module is associated with the preB cell complex, strongly suggesting that the mYL complex might be considered a “preB” receptor.
3.Immature B Cells are Selected Before Leaving the Bone Marrow
Before leaving the bone marrow, immature B cells that express surface IgM are confronted with the local “self” antigenic environment. They are particularly sensitive to triggering by multivalent antigens, resulting in their clonal deletion by apoptosis. Alternatively, soluble self antigens do not cause the immature B cells to die, but instead induce an anergic state that does not seem to prevent the cells migrating to the periphery. It should be noted, however, that this negative selection has a threshold that leaves a fraction of autoreactive cells going to the periphery. These cells are responsible for the presence in the bloodstream of natural autoantibodies that must be of some physiological relevance. Once validated by this “quality control,” immature B cells circulate to the periphery through blood vessels and lymphatic system, colonize the secondary lymphoid organs, and actively recirculate. They now have become mature B cells, expressing both IgM and IgD isotypes at their surface.
4.Final Steps of B-Cell Differentiation Take Place in the Periphery and are Antigen-Dependent
The B lymphocytes that are now circulating in the periphery are nondividing, short-lived cells. To achieve their ultimate differentiation, they must be triggered by an antigen, most often T-cell-dependent (Fig. 1). Within the first days after antigenic stimulation and T-cell help, activated B cells may mature to plasma cells that have an abundant endoplasmic reticulum and secrete immunoglobulins. Activated B cells may also evolve to colonize the primary follicle of a lymph node and generate a germinal center, where they interact with follicular dendritic cells, divide rapidly, switch to another isotype, most frequently IgG, and start to accumulate somatic hypermutations that considerably amplify diversity and affinity. Clones of high affinity will be positively selected by antigen and can now progress toward plasma cell differentiation or become long-lived B memory cells.
5.Conclusions
Differentiation of B cells is a highly sophisticated process that takes place continuously in the bone marrow and results in the constant emergence of a very large repertoire of immunoglobulins, expressed both as membrane B-cell receptors (BCRs) and soluble antibodies. The numerous molecular events that lead from the hematopoietic stem cell to the mature B cell are under constant selective pressure, implying a high level of cell death. Therefore, the available repertoire appears, at any given time, to be a compromise between the necessary economy in the gene number, compensated by the recombination processes, and an unavoidable wastage due to the stochastic aspects of gene rearrangements and to the negative selection of clones having a high affinity for self components.
References
P. D. Burrows and M. D. Cooper (1997) B cell development and differentiation. Curr. Opin. Immunol., 9, 239–244.
Y. J. Liu and C. Arpin (1997) Germinal center development. Immunol. Rev., 156, 111–126.
H. Karasuyama, A. Rolink, and F. Melchers (1996) Surrogate light chain on B cell development. Adv. Immunol, 63, 1–41.
A. Galy, M. Travis, D. Cen, and B. Chen (1995) Human T, B, natural killer and dendritic cells arise from a common bone marrow progenitor cell subset. Immunity 3, 459–473.
J. Banchereau and F. Rousset (1992) Human B lymphocytes: phenotype, proliferation, and differentiation. Adv. Immunol. 52, 125–262.
L. A. Herzenberg and A. B. Kantor (1993) B-cell lineages exist in the mouse. Immunol. Today 14, 79–83.



|
|
|
|
"عادة ليلية" قد تكون المفتاح للوقاية من الخرف
|
|
|
|
|
|
|
ممتص الصدمات: طريقة عمله وأهميته وأبرز علامات تلفه
|
|
|
|
|
|
|
ندوات وأنشطة قرآنية مختلفة يقيمها المجمَع العلمي في محافظتي النجف وكربلاء
|
|
|