


 الفيزياء الكلاسيكية
الفيزياء الكلاسيكية
 الكهربائية والمغناطيسية
الكهربائية والمغناطيسية
 علم البصريات
علم البصريات
 الفيزياء الحديثة
الفيزياء الحديثة
 النظرية النسبية
النظرية النسبية
 الفيزياء النووية
الفيزياء النووية
 فيزياء الحالة الصلبة
فيزياء الحالة الصلبة
 الليزر
الليزر
 علم الفلك
علم الفلك
 المجموعة الشمسية
المجموعة الشمسية
 الطاقة البديلة
الطاقة البديلة
 الفيزياء والعلوم الأخرى
الفيزياء والعلوم الأخرى
 مواضيع عامة في الفيزياء
مواضيع عامة في الفيزياء|
Read More
Date: 1-4-2021
Date: 8-5-2021
Date: 5-4-2021
|
Electrochemical energy
In electricity, one important form of potential energy exists in the atoms and molecules of some chemicals under special conditions.
Early in the history of electrical science, laboratory physicists found that when metals came into contact with certain chemical solutions, voltages appeared between the pieces of metal. These were the first electrochemical cells.
A piece of lead and a piece of lead dioxide immersed in an acid solution (Fig. 1) will show a persistent voltage. This can be detected by connecting a galvanometer between the pieces of metal. A resistor of about 1,000 ohms should always be used in series with the galvanometer in experiments of this kind; connecting the galvanometer directly will cause too much current to flow, possibly damaging the galvanometer and causing the acid to “boil.”
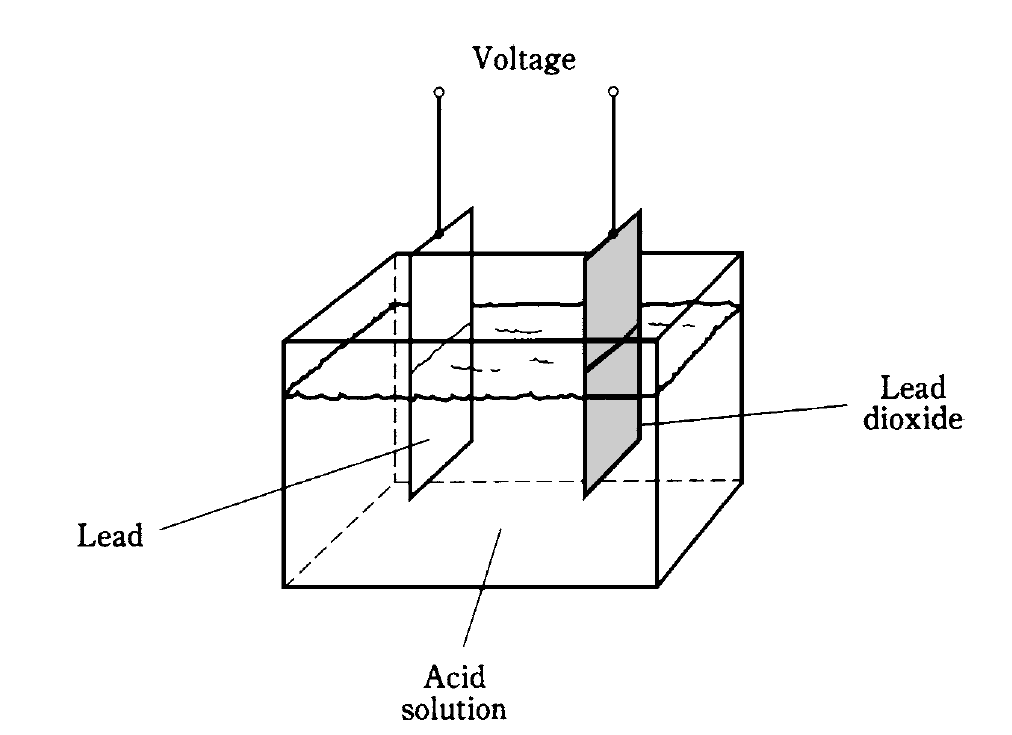
Fig. 1: Construction of a lead-acid electrochemical cell.
The chemicals and the metal have an inherent ability to produce a constant exchange of charge carriers. If the galvanometer and resistor are left hooked up between the two pieces of metal for a long time, the current will gradually decrease, and the electrodes will become coated. The acid will change, also. The chemical energy, a form of potential energy in the acid, will run out. All of the potential energy in the acid will have been turned into kinetic electrical energy as current in the wire and galvanometer. In turn, this current will have heated the resistor (another form of kinetic energy), and escaped into the air and into space.



|
|
|
|
"عادة ليلية" قد تكون المفتاح للوقاية من الخرف
|
|
|
|
|
|
|
ممتص الصدمات: طريقة عمله وأهميته وأبرز علامات تلفه
|
|
|
|
|
|
|
المجمع العلمي للقرآن الكريم يقيم جلسة حوارية لطلبة جامعة الكوفة
|
|
|