


 الفيزياء الكلاسيكية
الفيزياء الكلاسيكية
 الكهربائية والمغناطيسية
الكهربائية والمغناطيسية
 علم البصريات
علم البصريات
 الفيزياء الحديثة
الفيزياء الحديثة
 النظرية النسبية
النظرية النسبية
 الفيزياء النووية
الفيزياء النووية
 فيزياء الحالة الصلبة
فيزياء الحالة الصلبة
 الليزر
الليزر
 علم الفلك
علم الفلك
 المجموعة الشمسية
المجموعة الشمسية
 الطاقة البديلة
الطاقة البديلة
 الفيزياء والعلوم الأخرى
الفيزياء والعلوم الأخرى
 مواضيع عامة في الفيزياء
مواضيع عامة في الفيزياء|
Read More
Date: 3-9-2016
Date: 9-8-2016
Date: 25-7-2016
|
Heat Loss
An immersion heater of power J = 500 W is used to heat water in a bowl. After 2 minutes, the temperature increases from T1 = 85oC to T2 = 90°C. The heater is then switched off for an additional minute, and the temperature drops by ∆T = 1oC. Estimate the mass of the water in the bowl. The thermal capacity of water c = 4.2 × 103 J/kg K.
SOLUTION
Let τ1 = 2 min be the time the heater is operating. The energy added to the water and bowl will heat the water as well as the environment. We will assume that the heat loss Q1 to the surroundings is proportional to the elapsed time τ1 and that the changing temperature difference 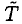 between the water and room temperature T ~ 23oC. During this phase, we may write
between the water and room temperature T ~ 23oC. During this phase, we may write
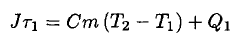 (1)
(1)
The heat loss Q1 is actually a time integral of some proportionality constant λ times the temperature difference as the water heats up. However, 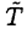 only varies by 5oC out of an average 65oC, so we will ignore the variation. The heat loss during the second phase is given by
only varies by 5oC out of an average 65oC, so we will ignore the variation. The heat loss during the second phase is given by
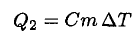 (2)
(2)
Just as in the heating phase, the heat loss is proportional to the elapsed time τ2 = τ1/2. Since 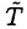 again only changes a little, we have
again only changes a little, we have
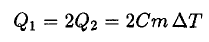 (3)
(3)
We may now eliminate Q1 from(1), yielding
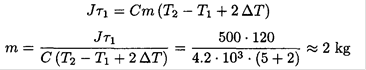



|
|
|
|
دراسة يابانية لتقليل مخاطر أمراض المواليد منخفضي الوزن
|
|
|
|
|
|
|
اكتشاف أكبر مرجان في العالم قبالة سواحل جزر سليمان
|
|
|
|
|
|
|
اتحاد كليات الطب الملكية البريطانية يشيد بالمستوى العلمي لطلبة جامعة العميد وبيئتها التعليمية
|
|
|