


 الفيزياء الكلاسيكية
الفيزياء الكلاسيكية
 الكهربائية والمغناطيسية
الكهربائية والمغناطيسية
 علم البصريات
علم البصريات
 الفيزياء الحديثة
الفيزياء الحديثة
 النظرية النسبية
النظرية النسبية
 الفيزياء النووية
الفيزياء النووية
 فيزياء الحالة الصلبة
فيزياء الحالة الصلبة
 الليزر
الليزر
 علم الفلك
علم الفلك
 المجموعة الشمسية
المجموعة الشمسية
 الطاقة البديلة
الطاقة البديلة
 الفيزياء والعلوم الأخرى
الفيزياء والعلوم الأخرى
 مواضيع عامة في الفيزياء
مواضيع عامة في الفيزياء|
Read More
Date: 14-8-2016
Date: 22-8-2016
Date: 28-7-2016
|
Chemical Potential of Ideal Gas
Derive the expression for the Gibbs free energy and chemical potential of N molecules of an ideal gas at temperature τ, pressure P, and volume V. Assume that all the molecules are in the electronic ground state with degeneracy g. At what temperature is this approximation valid?
SOLUTION
The expression for the Helmholtz free energy:
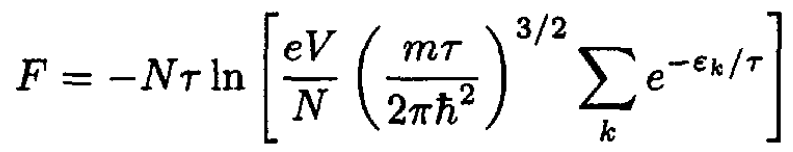 (1)
(1)
Since all the molecules are in the ground state, the sum only includes one term, which we can take as an energy zero, ε = 0. Then (1) becomes
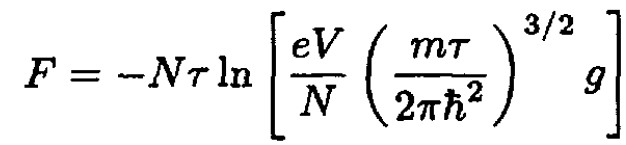 (2)
(2)
where we took into account a degeneracy of the ground state g. The Gibbs free energy G is then
 (3)
(3)
where we have expressed G as a function of τ, P. The chemical potential μ = G/N, so we obtain, from (3),
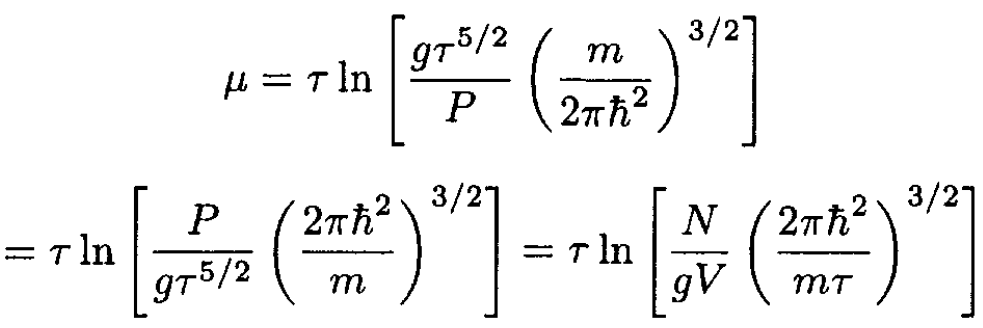 (4)
(4)
This approximation is valid when the temperature is much lower than the energy difference ∆E between the electronic ground state and the first excited state; since this ∆E is comparable to the ionization energy εion, this condition is equivalent to τ << εion. However, even at temperatures τ ~ εion, the gas is almost completely ionized. Therefore (4) is always valid for a nonionized gas.



|
|
|
|
تفوقت في الاختبار على الجميع.. فاكهة "خارقة" في عالم التغذية
|
|
|
|
|
|
|
أمين عام أوبك: النفط الخام والغاز الطبيعي "هبة من الله"
|
|
|
|
|
|
|
قسم شؤون المعارف ينظم دورة عن آليات عمل الفهارس الفنية للموسوعات والكتب لملاكاته
|
|
|