


 النبات
النبات
 الحيوان
الحيوان
 الأحياء المجهرية
الأحياء المجهرية
 علم الأمراض
علم الأمراض
 التقانة الإحيائية
التقانة الإحيائية
 التقنية الحيوية المكروبية
التقنية الحيوية المكروبية
 التقنية الحياتية النانوية
التقنية الحياتية النانوية
 علم الأجنة
علم الأجنة
 الأحياء الجزيئي
الأحياء الجزيئي
 علم وظائف الأعضاء
علم وظائف الأعضاء
 الغدد
الغدد
 المضادات الحيوية
المضادات الحيوية|
Read More
Date: 21-4-2021
Date: 27-2-2021
Date: 7-11-2020
|
Adenylate Cyclases
The ubiquity of cyclic AMP (cAMP) in regulating enzymatic activity and/or genetic expression in all kingdoms of life, except for the archaea (but see later), accounts for the interest displayed in its mode of synthesis and the vast amount of literature devoted to the enzymes that produce it, the adenylate cyclases. These enzymes, which catalyze synthesis of cAMP from ATP and yield pyrophosphate as a by-product, can be classified into four different classes according to their common features: (1) cyclases related to enterobacterial adenylate cyclases; (2) toxic adenylate cyclases isolated from bacterial pathogens; (3) a large and probably ancient class that comprises cyclases from both eukaryotes and prokaryotes and is strongly related to guanylate cyclases; and (4( one example, presently known only from the eubacteria Aeromonas hydrophila and yersinia pestis, that differs entirely from all other classes.
0.1.Class I: The Enterobacterial Type
The first complete adenylate cyclase gene, cya, was cloned and sequenced from Escherichia coli. Work on other enterobacteria, such as Erwinia chrysanthemi, Proteus mirabilis, Salmonella typhimurium, Yersinia intermedia, and Yersinia pestis demonstrates that both the environment of the genes and the proteins specified are similar in size and overall organization to those of E. coli at the corresponding locus (1). Analysis of the cya gene from other bacterial species, related to enterobacteria but distinct from them, using genetic complementation of appropriate cya defective strains of E. coli (and more recently by direct sequencing of whole genomes) reveals that the genes from many other bacteria, in particular Aeromonas caviae, Aeromonas hydrophila, Haemophilus influenzae, Pasteurella multocida, and Vibrio cholerae, directly synthesize a protein structurally and phylogenetically related to the E. coli cyclase (1, 2).
No long stretch of hydrophobic amino acid residues is present to explain the membrane-bound localization of the adenylate cyclases. In all cases, the proteins are very rich in cysteine residues, an uncommon feature for proteins located in the cytoplasm or at the cytoplasmic border of the membrane. This might account for the extreme difficulty in purifying the enzymes. In addition, they are also rich in histidine residues, which could indicate that metal ions take part in the folding and/or activity of the polypeptide chain, but no experimental data support this hypothesis. Finally, the protein is made of two functionally well-defined domains. The catalytic domain is NH2-terminal, whereas the glucose-sensitive regulatory domain is COOH-terminal. Comparison of the polypeptide sequence of the catalytic domain of the E. coli enzyme with sequences in the protein data libraries do not reveal significant identities with other known proteins. The catalytic domain sequence has been experimentally identified to be about 420 residues. Differences in the amino acid sequence of the E. chrysanthemi enzyme often result from the presence of complementary charged residues in place of neutral ones. This suggests that there are more electrostatic interactions (including salt bridges) that stabilize the protein at the lower growth temperature of this bacterium. This observation might be helpful when trying to understand the tertiary structure of the protein, which is still not known.
The carboxy-terminal domain of the protein is involved in regulating of the enzymatic activity, in particular its inhibition by glucose. A component of the phosphorylation cascade that mediates import of glucose in the cell, enzyme IIAGlc, is involved in this regulation, but in a way not yet understood. An aspartate residue (Asp414 in the E. coli enzyme) is involved in the process in an unknown way. Tonic inhibition of the catalytic domain by the regulatory domain could be relieved by phosphorylation of this residue, although such phosphorylation has never been demonstrated (3).
0.2. Class II: The Calmodulin-Activated Toxic Class
Whooping cough is caused by the gram-negative bacterium Bordetella pertussis, which secretes many toxic proteins into the medium, including an adenylate cyclase. In 1980 it was discovered that this enzyme is activated by a host protein, calmodulin, which does not occur in bacteria (4). Two years later, Leppla (5) demonstrated that another toxic adenylate cyclase, secreted by a gram-positive bacterium, Bacillus anthracis, the etiological agent of anthrax, is also activated by host calmodulin. These observations stimulated intense efforts, but several years were required before the cya genes from either organisms could be cloned. However, in 1988 a simple idea, predating its generalization under the name “two-hybrid system,” in vivo complementation by a plasmid encoding an activator of the function (in this case, calmodulin), permitted the cloning of adenylate cyclase genes coding for the calmodulin-dependent cyclases (6).
Bordetella pertussis adenylate cyclase is synthesized as a large bifunctional polypeptide chain of 1706 amino acid residues. This contrasts with the various low values reported for the molecular weight of the purified protein (from 43 to 70 kDa). The explanation became apparent when it was demonstrated that the N-terminal segment of the protein (400 residues) alone displays calmodulin-activated adenylate cyclase activity, whereas the rest of the molecule is responsible for hemolytic activity and for transporting the toxin. Sequence, molecular genetic, and physiological studies indicate that the adenylate cyclase domain is fused to a polypeptide chain similar to that of the E. coli hemolysin toxin. Therefore the name cyclolysin was coined for the toxic adenylate cyclase from B. pertussis.
The adenylate cyclase of B. anthracis has been named after the symptom it triggers in the infected host, edema factor. It is encoded in a plasmid, together with another toxin, the lethal factor and a carrier protein, the protective antigen, necessary to internalize both the edema factor and the lethal factor into host target cells. The adenylate cyclase (edema factor) protein, 800 amino acid residues long, comprises four regions of different function. The first region is a signal peptide, permits secretion of the protein. The second region corresponds to the domain that binds with the protective antigen. The third region encodes the adenylate cyclase function. It is followed by the fourth region of unknown function. These toxic adenylate cyclases have been subjected to a most thorough biochemical analysis, but they have not yet been crystallized.
In spite of several attempts to isolate other members of this class, until 1998, we knew only three examples of such proteins, isolated from extremely distant bacteria, one gram-positive and two gram-negative (the adenylate cyclase from B. bronchiseptica is very similar to the B. pertussis enzyme) (7). Several examples of similar proteins have now been discovered in Pseudomonas aeruginosa, and in Yersinia species. Comparison of the catalytic regions of the B. pertussis and B. anthracis adenylate cyclases identified four conserved regions that are involved in catalysis, calmodulin binding and activation. The first region comprises a sequence, Gly-XXXX-Gly(Ala)-Lys-Ser, similar to the nucleotide-binding motif found in many ATP- or GTP-binding proteins.
Therefore it was proposed as part of the catalytic site, and in vitro mutagenesis substantiated this interpretation. A second region, with the sequence Pro-Leu-Thr-Ala-Asp-Ile-Asp having some similarity in 6-phosphofructokinase, is also involved in catalysis, and it was proposed that the aspartate residues present in this region are involved in binding ribose and magnesium-phosphate. Although it is strongly conserved, however, the first proline residue does not seem very important because it could be replaced by a leucine residue without any measurable influence on the activity or calmodulin activation of the wild-type enzyme.
Although many calmodulin-dependent enzymes have been identified, the mechanism of activation by calmodulin is still poorly understood (see Calmodulin). In several cases, limited proteolysis releases active, calmodulin-independent forms of the enzymes. Accordingly, it was proposed that the calmodulin-binding domain of these enzymes blocks access of substrates to the active site and that activation results because an inhibitory domain is removed upon binding calmodulin. The most original feature of the B. pertussis protein is that it can be split into two separate domains which recover most of the initial activity when combined. This observation, together the with analysis of mutants in the region conserved between the B. anthracis and B. pertussis enzymes, indicates that these proteins may form a catalytic center from the cooperation of two halves. The function of calmodulin may be to trigger the appropriate conformational movement necessary to form an active catalytic center (3).
0.3. Class III: The “Universal” Class
Adenylate cyclases from multicellular eukaryotes have long remained elusive because purifying the corresponding catalytic subunit is extremely difficult. Following intense work all over the world, however, they have been the first adenylate cyclases to be crystallized and analyzed by X-ray crystallography (see Fig. 1). The activity of these enzymes is subject to a complex regulatory pattern, in particular by GTP-binding proteins. Class III enzymes were first discovered in yeast, but, for convenience, we start with the eubacterial enzymes.
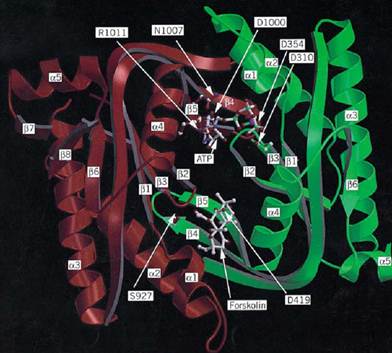
Figure 1. Three-dimensional model of the C1 and C2 domains of the catalytic core of type I adenylate cyclase derived fr the X-ray crystallographic structure of the C2 homodimer of the type II enzyme (19). The polypeptide backbone of the Cdomain is green and that of C2 is red. The C-terminal nonhomologous segment of C1 is truncated after the a-helix 5 (low right). Only a few side chains in the active site are shown, designated by one-letter abbreviations. When they differed fro the residues in the crystal structure, they were placed according to Liu et al. [Proc. Natl. Acad. Sci. (1997) 94, 1341413419-]ATP was placed as described by Liu et al. and modified according to the interactions reported by Tesmer et al. [Science (1997) 278, 1907–1916.] The ligand atoms are indicated by C, white; N, blue; O, red; P, green, and Mg, purple. figure is kindly provided by James Hurley.
0.3.1. Eubacteria
Class III adenylate cyclases form a very diverse collection in eubacteria, both in length and in regulation. Gram-positive bacteria such as Corynebacterium liquefaciens secrete large amounts of cAMP because they have a very active class III adenylate cyclase, which is activated by pyruvate. S. coelicolor synthesizes a much less active adenylate cyclase involved in aeromycelium formation. Gram-negative bacteria, such as Rhizobium meliloti, synthesize at least two different adenylate cyclases. Disruption of both genes simultaneously does not alter cAMP production and suggests the presence of further enzymes. The gram-negative sliding myxobacterium Stigmatella aurantiaca, that exhibits an elaborated differentiation pattern, harbors at least two adenylate cyclase genes, each of them corresponding to class III enzymes. They have been partially purified and are inhibited by adenosine, as are the mammalian enzymes (see later). They comprise two domains. The catalytic domain is carboxy-terminal and the regulatory domain is a likely ion transporter in one case and the phosphorylated moiety of a two-component regulatory system in the other. Many other bacteria possess class III cyclases, in particular cyanobacteria (8, 9). These enzymes generally comprise two domains. The catalytic domain is carboxy-terminal. There are no indications that they must oligomerize to be active.
0.3.2. Lower Eukaryotes
Saccharomyces cerevisiae was the first organism from which class III adenylate cyclase genes were cloned and sequenced. The enzyme is activated by the RAS gene product (10, 11). Two forms of the enzyme may exist. A long form contains repetitions of a leucine-rich motif that plays a regulatory role and whose significance was recently substantiated and extended. Then it became clear that this eukaryotic adenylate cyclase is completely different from the enterobacterial class because of sequence differences in the catalytic center and also because the organization of the gene is different. The catalytic domain is located at the COOH-terminus in S. cerevisiae cyclase, whereas it is found at the NH2-terminus in E. coli. The yeast enzyme remained the only example of its class until Garbers, Goeddel and co-workers (12) recognized that the genes encoding guanylate cyclases that had been cloned from several metazoans were derived from an ancestor common to the yeast adenylate cyclase. Another member of class III was subsequently discovered by Young et al. (13), who cloned the adenylate cyclase gene from Schizosaccharomyces pombe by hybridization using the catalytic domain gene sequence from S. cerevisiae as a probe. Finally, the first higher eukaryote adenylate cyclase gene isolated in Gilman's laboratory (14) from bovine brain displayed features clearly reminiscent of this class. Since then, many other genes or cDNA for adenylate cyclases belonging to this class have been isolated and sequenced from lower eukaryotes: Saccharomyces kluyveri, Trypanosoma brucei and T. equiperdum, Plasmodium falciparum, Neurospora crassa, and Dictyostelium discoideum (3).
0.3.3. Higher Eukaryotes: Nine Types
Many class III adenylate cyclases have been identified in higher eukaryotes, in particular in vertebrates, but the most thorough study is in mammals, where several types differing in their regulatory properties have been identified (15). All are regulated in more or less complex ways by G-proteins (16). Mammalian adenylate cyclases are informally grouped into nine types according to their tissue location and activity regulation. All but type 9 are activated by the diterpene forskolin, and some are activated by protein kinase C and/or other regulators. Type 1 enzymes were described as calmodulin-activated enzymes from brain. Type 2 proteins are found in brain, lung and other tissues. Type 3 are abundant in olfactory tissue, and the smaller type 4 enzymes are present in testicular tissue. Adenylate cyclase 1, 2, and 8 are positively regulated by calcium/calmodulin, whereas types 5 and 6 are directly inhibited by calcium. Adenylate cyclase 2 and 4 are sensitive to multiple regulatory effects from diverse receptors (3, 15). Adenylate cyclase 9 mRNA, found in rat brain, is particularly abundant in the hippocampus, cerebellum, and neocortex (17). However, the classification into types is somewhat arbitrary (for example, type 4 can also be calmodulin-activated). They all have overall similar structures. Two phylogenetically related cytoplasmic domains are required for catalysis. All types are connected by an integral membrane domain and have variable integral membrane domains at the NH2 terminus of the protein. Among their many functions, their role in synaptic plasticity and memory is particularly interesting (18) and will certainly make adenylate cyclases extremely fashionable again.
0.3.4. Three-Dimensional Structure
The diverse origins of class III adenylate cyclases are reflected in the wide variation in their general organizations and molecular weights. The smallest protein is the R. meliloti enzyme, which contains only a catalytic domain, although some data suggest that an upstream sequence may yield a much longer protein that has a complex regulatory pattern. The next shortest protein, also bacterial, is the enzyme from C. liquifaciens. The yeasts produce long proteins, as do higher eukaryotes (except in the case of the testicular enzyme). In all cases, the catalytic domain is located at the COOH-terminus. The mammalian enzymes consist of twelve hydrophobic membrane-spanning regions that form two distinct domains, two cytoplasmic regions that contain both variable and conserved regions, and, in particular, two well-conserved domains that are responsible for catalysis.
Comparison of the catalytic domain sequences of the class III proteins shows that four amino acid stretches are strongly conserved: (1) (Met/Leu/Ile/Val)-(Met/Leu/Ile/Val)-Phe-(Ala/Thr)-(Asp/Ser)-(Leu/Ile)-X-(Asn/Asp)-(Phe/Ser); (2) (Ile/Val)-Lys-Thr-X-Gly-(Ser/Asp)-(Ala/Ser/Thr)-(Tyr/Phe)- Met; (3) (Met/Leu/Ile/Val)-(Arg/Lys)-(Met/Leu/Ile/Val)-Gly-(Met/Leu/Ile/Val)-(His/Asn)-X-Gly-X-(Val/Ala)-(Val/Leu)-(Ala/Ser)-Gly; and (4) (Trp/Tyr/Phe)-Gly-(Asn/Asp/Pro)-Thr-Val-Asn-X-Ala-Ser-Arg-(Met/Leu/Ile/Val) (see Fig. 2). Crystallization of the catalytic core of one protein Met; (3) (Met/Leu/Ile/Val)-(Arg/Lys)-(Met/Leu/Ile/Val)-Gly-(Met/Leu/Ile/Val)-(His/Asn)-X-Gly-X-(Val/Ala)-(Val/Leu)-(Ala/Ser)-Gly; and (4) (Trp/Tyr/Phe)-Gly-(Asn/Asp/Pro)-Thr-Val-Asn-X-Ala-Ser-Arg-(Met/Leu/Ile/Val) (see Fig. 2). Crystallization of the catalytic core of one protein and determination of its structure by X-ray diffraction (19) showed that these regions are part of the organization of the structure (Fig. 1). The crystal structure contains the forskolin-binding site but unfortunately not the nucleotide binding site. As a step toward understanding the evolution and function of class III cyclases, enzymes displaying significant guanylyl cyclase activity that have evolved from an adenylyl cyclase ancestor were isolated. A single amino acid residue change (Gly-Asp-Thr-Val-Asn to Gly-Asp-Thr-Ile-Asn in the region of the fourth a-helix of the catalytic core) alters the nucleotide specificity of the enzyme (20). This corresponds to a pocket situated in a region of the protein that might accommodate the heterocyclic base (19).
determination of its structure by X-ray diffraction (19) showed that these regions are part of the organization of the structure (Fig. 1). The crystal structure contains the forskolin-binding site but unfortunately not the nucleotide binding site. As a step toward understanding the evolution and function of class III cyclases, enzymes displaying significant guanylyl cyclase activity that have evolved from an adenylyl cyclase ancestor were isolated. A single amino acid residue change (Gly-Asp-Thr-Val-Asn to Gly-Asp-Thr-Ile-Asn in the region of the fourth a-helix of the catalytic core) alters the nucleotide specificity of the enzyme (20). This corresponds to a pocket situated in a region of the protein that might accommodate the heterocyclic base (19).

Figure 2. Alignment of the amino acid sequences of Class III adenylate cyclases from various organisms. The four regio text are enclosed in the four boxes. Secondary structural elements found in the crystal structure of Fig. 1 are displayed uncorresponding residues of the active site depicted in Fig. 1 are indicated.
0.4. Class IV Adenylate Cyclases
The preceding three classes of structurally unrelated adenylate cyclases already pose a challenging problem. So it was a surprise that Aeromonas hydrophila synthesizes another enzyme, a very small cyclase of 193 residues, which has an optimal temperature for activity of 65°C and is at least ten times more active than the class I adenylate cyclase in the same organism (21). No function has yet been discovered for this protein. As yet, it has been found only in various isolates of A. hydrophila and in Y. pestis (unpublished). There was one report of the presence of cAMP in Archaea, but this was later proven to be the result of an artifact of the growth culture. Therefore it was interesting to see that the sequence of adenylate cyclase from A. hydrophila is significantly similar to a gene product of the archaebacterium Methanococcus jannaschii. The gene is expressed in E. coli, where it is toxic, but it does not restore cAMP synthesis. Therefore, nothing is yet known about the nature of adenylate cyclases, if they exist, in Archaea, but we may expect that, at some point, they might be discovered and that they would belong to this new class.
References
1. A. Danchin (1993) Adv. Sec. Mess. Phosphoprot. Res., 27, 109–161.
2. P. Trotot, O. Sismeiro, C. Vivarès, P. Glaser, A. Bresson-Roy, and A. Danchin (1996)
Biochimie 78, 277–287.
3. O. Barzu and A. Danchin (1994) Prog. Nucleic Acid Res. Mol. Biol. 49, 241–283.
4. J. Wolff, G. H. Cook, A. R. Goldhammer, and S. A. Berkowitz (1980) Proc. Natl Acad. Sci.
USA 77, 3840–3844.
5. S. H. Leppla (1982) Proc. Natl Acad. Sci. USA 79, 3162–3166.
6. P. Glaser, D. Ladant, O. Sezer, F. Pichot, A. Ullmann, and A. Danchin (1988) Mol. Microbiol.
2, 19–30.
7. F. Betsou, O. Sismeiro, A. Danchin, and N. Guiso (1995) Gene 162, 165–166.
8. M. Kasahara, K. Yashiro, T. Sakamoto, and M. Ohmori (1997) Plant Cell. Physiol. 38, 828–
836.
9. M. Katayama and M. Ohmori (1997) J. Bacteriol. 179, 3588–3593.
10. T. Kataoka, D. Broek, and M. Wigler (1985) Cell 43, 493–505.
11. P. Masson, G. Lenzen, J. M. Jacquemin, and A. Danchin (1986) Curr. Genet. 10, 343–352.
12. M. Chinkers, D. L. Garbers, M. S. Chang, D. G. Lowe, H. Chin, D. V. Goeddel, and S. Schulz (1989) Nature 338, 78–83.
13. D. Young, M. Riggs, J. Field, A. Vojtek, D. Broek, and M. Wigler (1989) Proc. Natl. Acad. Sci. USA 86, 7989–7993.
14. J. Krupinski, F. Coussen, H. A. Bakalyar, W.-J. Tang, P. G. Feinstein, K. Orth, C. Slaughter, R. R. Reed, and A. G. Gilman (1989) Science 244, 1558–1564.
15. J. Hanoune, Y. Pouille, E. Tzavara, T. Shen, L. Lipskaya, N. Miyamoto, Y. Suzuki, and N. Defer (1997) Mol. Cell. Endocrinol. 128, 179–194.
16. A. Marjamaki, M. Sato, R. Bouet-Alard, Q. Yang, I. Limon-Boulez, C. Legrand, and S. M. Lanier (1997) J. Biol. Chem. 272, 16466–16473.
17. R. T. Premont, I. Matsuoka, M. G. Mattei, Y. Pouille, N. Defer, and J. Hanoune (1996) J. Biol. Chem. 271, 13900–13907.
18. M. D. Nielsen, G. C. K. Chan, S. W. Poser, and D. R. Storm (1996) J. Biol. Chem. 271, 3330833316- .
19. G. Zhang, Y. Liu, A. E. Ruoho, and J. H. Hurley (1997) Nature 386, 247–253.
20. A. Beuve, E. Krin, and A. Danchin (1993) Compt. Rend. Acad. Sci. Paris 316, 553–559.
21. O. Sismeiro, P. Trotot, F. Biville, C. Vivarès, and A. Danchin (1998) J. Bacteriol. 180, 3339–3344.



|
|
|
|
دراسة يابانية لتقليل مخاطر أمراض المواليد منخفضي الوزن
|
|
|
|
|
|
|
اكتشاف أكبر مرجان في العالم قبالة سواحل جزر سليمان
|
|
|
|
|
|
|
اتحاد كليات الطب الملكية البريطانية يشيد بالمستوى العلمي لطلبة جامعة العميد وبيئتها التعليمية
|
|
|