


 النبات
النبات
 الحيوان
الحيوان
 الأحياء المجهرية
الأحياء المجهرية
 علم الأمراض
علم الأمراض
 التقانة الإحيائية
التقانة الإحيائية
 التقنية الحيوية المكروبية
التقنية الحيوية المكروبية
 التقنية الحياتية النانوية
التقنية الحياتية النانوية
 علم الأجنة
علم الأجنة
 الأحياء الجزيئي
الأحياء الجزيئي
 علم وظائف الأعضاء
علم وظائف الأعضاء
 الغدد
الغدد
 المضادات الحيوية
المضادات الحيوية|
Read More
Date: 7-11-2020
Date: 8-5-2016
Date: 25-4-2016
|
Nucleosomes Are Displaced and Reassembled During Transcription
KEY CONCEPTS
-Most transcribed genes retain a nucleosomal structure, though the organization of the chromatin changes during transcription.
-Some heavily transcribed genes appear to be exceptional cases that are devoid of nucleosomes.
-RNA polymerase displaces histone octamers during transcription in vitro, but octamers reassociate with DNA as soon as the polymerase has passed.
-Nucleosomes are reorganized when transcription passes through a gene.
-Additional factors are required for RNA polymerase to displace octamers during transcription and for the histones to reassemble into nucleosomes after transcription.
Heavily transcribed chromatin adopts structures that are visibly too extended to still be contained in nucleosomes. In the intensively transcribed genes encoding rRNA shown in FIGURE 1, the extreme packing of RNA polymerases makes it difficult to see the DNA. Researchers cannot directly measure the lengths of the rRNA transcripts because the RNA is compacted by proteins, but we know (from the sequence of the rRNA) how long the transcript
must be. The length of the transcribed DNA segment, which is measured by the length of the axis of the “Christmas tree” shape shown, is about 85% of the length of the pre-rRNA. This means that the DNA is almost completely extended.

FIGURE 1. Individual rDNA transcription units alternate with nontranscribed DNA segments.
Reproduced from: Miller, O. L., and BeattyB. R. 1969. Science 164:955–957. Photo courtesy of Oscar Miller.
On the other hand, Researchers can extract transcriptionally active complexes of SV40 minichromosomes from infected cells. They contain the usual complement of histones and display a beaded structure. Chains of RNA can extend from the minichromosome, as shown in FIGURE 2. This argues that transcription can proceed while the SV40 DNA is organized into nucleosomes. Of course, the SV40 minichromosome is transcribed less intensively than the rRNA genes.

FIGURE 2. An SV40 minichromosome is transcribed while maintaining a nucleosomal structure.
Reprinted from: Gariglio, P., et al. 1979. “The template of the isolated native.” J Mol Bio 131:75–105, with permission from Elsevier (http://www.sciencedirect.com/science/journal/00222836). Photo courtesy of Pierre Chambon, College of France.
Transcription involves the unwinding of DNA, thus it seems obvious that some “elbow room” must be needed for the process. In thinking about transcription, we must keep in mind the relative sizes of RNA polymerase and the nucleosome. Eukaryotic RNA polymerases are large multisubunit proteins, typically greater than 500 kilodaltons (kD). Compare this with the approximately 260 kD of the nucleosome. FIGURE 3 illustrates the relative sizes of RNA polymerase and the nucleosome. Consider the two turns that DNA makes around the nucleosome. Would RNA polymerase have sufficient access to DNA if the nucleic acid were confined to this path? During transcription, as RNA polymerase moves along the template, it binds tightly to a region of about 50 bp, including a locally unwound segment of about 12 bp. The need to unwind DNA makes it seem unlikely that the segment engaged by RNA polymerase could remain on the surface of the histone octamer.
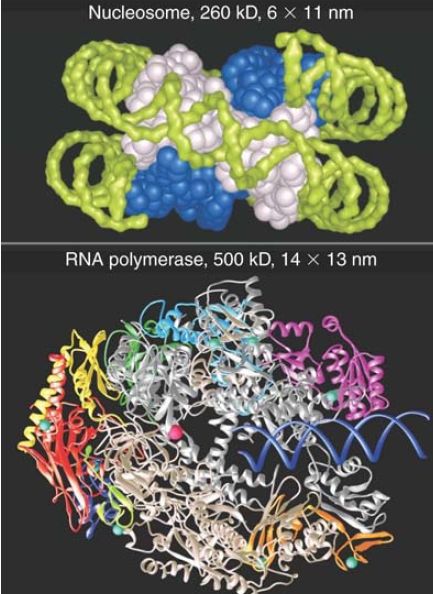
FIGURE 3. RNA polymerase is nearly twice the size of the nucleosome and might encounter difficulties in following the DNA around the histone octamer.
Top photo courtesy of E. N. Moudrianakis, the Johns Hopkins University. Bottom photo courtesy of Roger Kornberg, Stanford University School of Medicine.
It therefore seems inevitable that transcription must involve a structural change. Thus, the first question to ask about the structure of active genes is whether DNA being transcribed remains organized in nucleosomes. Experiments to test whether an RNA polymerase can transcribe directly through a nucleosome suggest that the histone octamer is displaced by the act of transcription.

FIGURE 4 shows what happens when the phage T7 RNA polymerase transcribes a short piece of DNA containing a single octamer core in vitro. The core remains associated with the DNA after the polymerase passes, but it is found in a different location.
The core is most likely to rebind to the same DNA molecule from which it was displaced. Crosslinking the histones within the octamer does not create an obstacle to transcription, suggesting that (at least in vitro) transcription does not require dissociation of the octamer into its component histones.
FIGURE 4 An experiment to test the effect of transcription on nucleosomes shows that the histone octamer is displaced from DNA and rebinds at a new position.
Thus a small RNA polymerase can displace a single nucleosome, which reforms behind it, during transcription. Of course, the situation is more complex in a eukaryotic nucleus. Eukaryotic RNA polymerases are much larger, and the impediment to progress is a string of connected nucleosomes (which can also be folded into higher-order structures). Overcoming these obstacles requires additional factors that act on chromatin .
The organization of nucleosomes can be dramatically changed by transcription. This is easiest to observe in inducible genes that have distinct on and off states under different conditions. In many cases, before activation a gene might display a single dominant pattern of nucleosomes that are organized from the promoter and throughout the coding region. When the gene is activated, the nucleosomes become highly mobilized and adopt a number of alternative positions. One or a few nucleosomes might be displaced from the promoter region, but overall nucleosomes typically remain present at a similar density. (However they are no longer organized in phase.) The action of ATP-dependent chromatin remodelers and histone modifiers are typically required to alter the nucleosomal positioning . When repression is reestablished, positioning reappears.
The unifying model is to suppose that RNA polymerase, with the assistance of chromatin remodelers, displaces histone octamers (either as a whole, or as dimers and tetramers) as transcription progresses. If the DNA behind the polymerase is available, the nucleosome is reassembled there. If the DNA is not available—for example, because another polymerase continues immediately behind the first—the octamer might be permanently displaced, and the DNA might persist in an extended form.
Other factors that are critical during transcription elongation, when nucleosomes are being rapidly displaced and reassembled, have been identified. The first of these to be characterized is a heterodimeric factor called FACT (facilitates chromatin transcription), which behaves like a transcription elongation factor. FACT is not part of RNA polymerase; however, it associates with it specifically during the elongation phase of transcription. FACT consists of two subunits that are well conserved in all eukaryotes, and it is associated with the chromatin of active genes.
When FACT is added to isolated nucleosomes, it causes them to lose H2A-H2B dimers. During transcription in vitro, it converts nucleosomes to “hexasomes” that have lost H2A-H2B dimers. This suggests that FACT is part of a mechanism for displacing octamers during transcription. FACT may also be involved in the reassembly of nucleosomes after transcription, because it assists formation of nucleosomes from core histones, thus acting like a histone chaperone. There is evidence in vivo that H2A-H2B dimers are displaced more readily during transcription than H3-H4 tetramers, suggesting that tetramers and dimers can be reassembled sequentially after transcription as they are after passage of a replication fork .
This suggests a model like that shown in FIGURE 5, in which FACT (or a similar factor) detaches H2A-H2B from a nucleosome in front of RNA polymerase and then helps to add it to a nucleosome that is reassembling behind the enzyme. Other factors are likely to be required to complete the process. FACT’s role might be more complex than this, because FACT has also been implicated in transcription initiation and replication elongation. Another intriguing model that has been proposed is that FACT stabilizes a “reorganized” nucleosome, in which the dimers and tetramer remain locally tethered via FACT but are not stably organized into a canonical nucleosome. The model presumes the H2A-H2B dimers are less stable in this reorganized state, and thus more easily displaced. In this state, the nucleosomal DNA is highly accessible, and the reorganized nucleosome can either revert to the stable canonical organization or be displaced as needed for transcription.
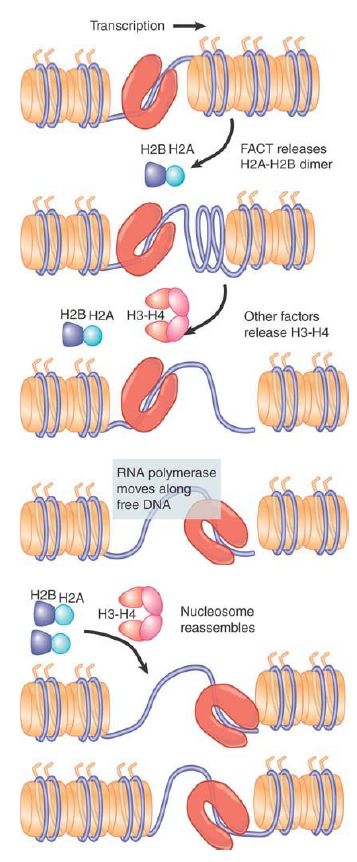
FIGURE 5. Histone octamers are disassembled ahead of transcription to remove nucleosomes. They re-form following transcription. Release of H2A-H2B dimers probably initiates the disassembly process.
Several other factors have been identified that play key roles in either nucleosome displacement or reassembly during transcription. These include the Spt6 protein, a factor involved in “resetting” chromatin structure after transcription. Spt6, like FACT, colocalizes with actively transcribed regions and can act as a histone chaperone to promote nucleosome assembly. Although CAF-1 is known to be involved only in replication-dependent histone deposition, one of CAF-1′s partners in replication might in fact play a role in transcription, as well. The CAF-1–associated protein Rtt106 is an H3-H4 chaperone that has recently been shown to play a role in H3 deposition during transcription.



|
|
|
|
تفوقت في الاختبار على الجميع.. فاكهة "خارقة" في عالم التغذية
|
|
|
|
|
|
|
أمين عام أوبك: النفط الخام والغاز الطبيعي "هبة من الله"
|
|
|
|
|
|
|
قسم شؤون المعارف ينظم دورة عن آليات عمل الفهارس الفنية للموسوعات والكتب لملاكاته
|
|
|