


 النبات
النبات
 الحيوان
الحيوان
 الأحياء المجهرية
الأحياء المجهرية
 علم الأمراض
علم الأمراض
 التقانة الإحيائية
التقانة الإحيائية
 التقنية الحيوية المكروبية
التقنية الحيوية المكروبية
 التقنية الحياتية النانوية
التقنية الحياتية النانوية
 علم الأجنة
علم الأجنة
 الأحياء الجزيئي
الأحياء الجزيئي
 علم وظائف الأعضاء
علم وظائف الأعضاء
 الغدد
الغدد
 المضادات الحيوية
المضادات الحيوية|
Read More
Date:
Date: 21-4-2021
Date: 28-4-2016
|
Cloning Vectors Can Be Specialized for Different Purposes
KEY CONCEPTS
-Cloning vectors can be bacterial plasmids, phages, cosmids, or yeast artificial chromosomes.
-Shuttle vectors can be propagated in more than one type of host cell.
-Expression vectors contain promoters that allow transcription of any cloned gene.
-Reporter genes can be used to measure promoter activity or tissue-specific expression.
-Numerous methods exist to introduce DNA into different target cells.
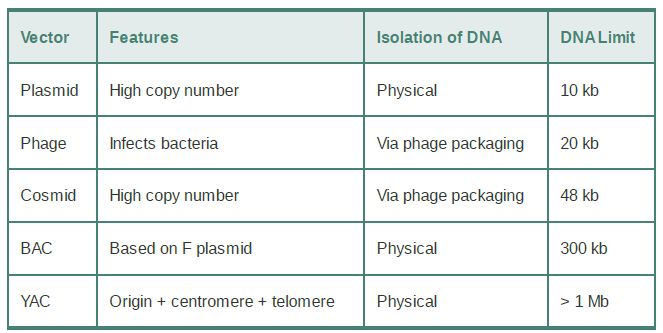
In the example , we described the use of a vector that is designed simply for amplifying insert DNA, with inserts up to ~10 kb. It is often desirable to clone larger inserts, though, and sometimes the goal is not just to amplify the DNA but also to express cloned genes in cells, investigate properties of a promoter, or create various fusion proteins (defined shortly). TABLE .1 summarizes the properties of the most common classes of cloning vectors. These include vectors based on bacteriophage genomes, which can be used in bacteria but have the disadvantage that only a limited amount of DNA can be packaged into the viral coat (although more than can be carried in a plasmid). The advantages of plasmids and phages are combined in the cosmid, which propagates like a plasmid but uses the packaging mechanism of phage lambda to deliver the DNA to the bacterial cells. Cosmids can carry inserts of up to 47 kb (the
maximum length of DNA that can be packaged into the phage head).
TABLE .1 Cloning vectors may be based on plasmids or phages or may mimic eukaryotic chromosomes.
Two vectors used for cloning the largest possible DNA inserts are the yeast artificial chromosome (YAC) and the human artificial chromosome (HAC). A YAC has a yeast origin to support replication, a centromere to ensure proper segregation, and telomeres to afford stability. In effect, it is propagated just like a yeast chromosome and can carry inserts measured in the megabase (Mb) length range. The HAC is the newest addition to the line of vectors and it offers the advantage of having virtually unlimited capacity. There is an extremely useful class of vectors known as shuttle vectors that we can use in more than one species of host cell. The example shown in FIGURE 1 contains origins of replication and selectable markers for both E. coli and the yeast Saccharomyces cerevisiae. It can replicate as a circular multicopy plasmid in E.coli. It has a yeast centromere, and it also has yeast telomeres adjacent to BamHI restriction sites so that cleavage with BamHI generates a YAC that can be propagated in yeast.
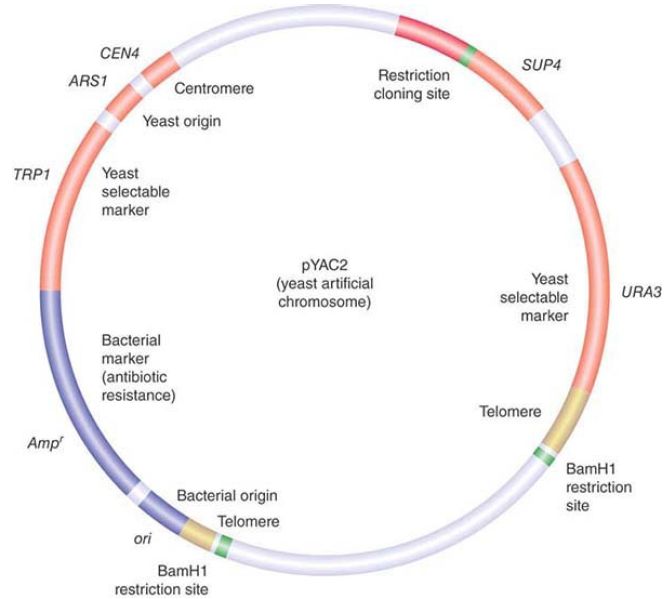
FIGURE 1. pYAC2 is a cloning vector with features to allow replication and selection in both bacteria and yeast. Bacterial features (shown in blue) include an origin of replication and antibiotic resistance gene. Yeast features (shown in red and yellow) include an origin, centromere, two selectable markers, and telomeres.
Other vectors, such as expression vectors, can contain promoters to drive expression of genes. Any open reading frame can be inserted into the vector and expressed without further modification. These promoters can be continuously active, or they can be inducible so that they are only expressed under specific conditions.
Alternatively, the goal might be to study the function of a cloned promoter of interest in order to understand the normal regulation of a gene. In this case, rather than using the actual gene, we can use an easily detected reporter gene under control of the promoter of interest.
The type of reporter gene that is most appropriate depends on whether we are interested in quantitating the efficiency of the promoter (and, for example, determining the effects of mutations in it or the activities of transcription factors that bind to it) or determining its tissue-specific pattern of expression. FIGURE 2. summarizes a common system for assaying promoter activity. A cloning vector is created that has a eukaryotic promoter linked to the coding region of luciferase, a gene that encodes the enzyme responsible for bioluminescence in the firefly. In general, a transcription termination signal is added to ensure the proper generation of the mRNA. The hybrid vector is introduced into target cells, and the cells are grown and subjected to any appropriate experimental treatments. The level of luciferase activity is measured by addition of its substrate luciferin. Luciferase activity results in light emission that can be measured at 562 nanometers (nm) and is directly proportional to the amount of enzyme that was made, which in turn depends upon the activity of the promoter.
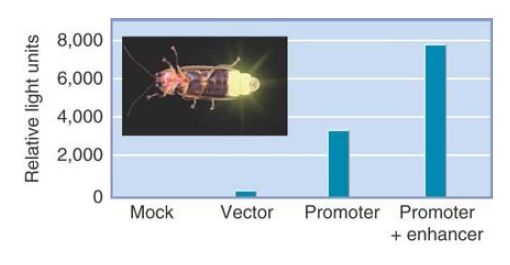
FIGURE 2. Luciferase (derived from fireflies such as the one shown here) is a popular reporter gene. The graph shows the results from mammalian cells transfected with a luciferase vector driven by a minimal promoter or the promoter plus a putative enhancer. The levels of luciferase activity correlate with the activities of the promoters.
Some very striking reporters are now available for visualizing gene expression. The lacZ gene, described in the blue/white selection strategy earlier, also serves as a very useful reporter gene.
FIGURE 3 shows what happens when the lacZ gene is placed under the control of a promoter that regulates the expression of a gene in the nervous system. The tissues in which this promoter is normally active can be visualized by providing the X-gal substrate to stain the embryo.
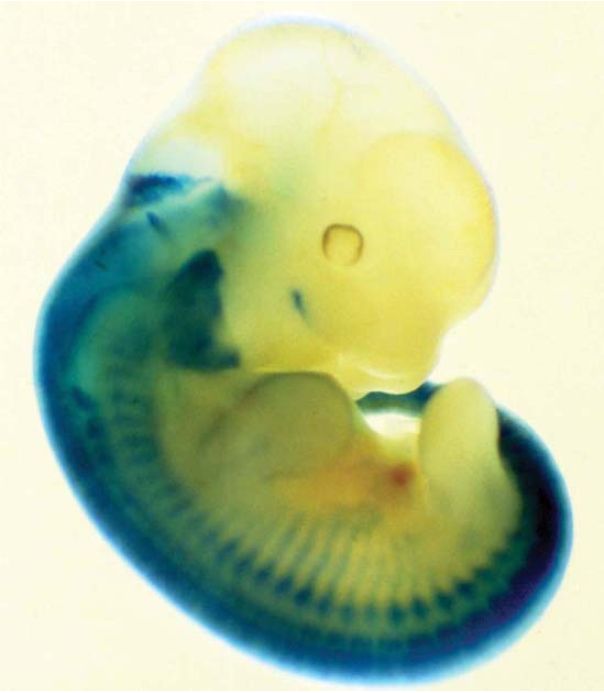
FIGURE 3 Expression of a lacZ gene can be followed in the mouse by staining for β-gal (in blue). In this example, lacZ was expressed under the control of a promoter of a mouse gene that is expressed in the nervous system. The corresponding tissues can be visualized by blue staining.
Photo courtesy of Robb Krumlauf, Stowers Institute for Medical Research. One of the most popular reporters that can be used to visualize patterns of gene expression is green fluorescent protein (GFP), which is obtained from jellyfish. GFP is a naturally fluorescent protein that, when excited with one wavelength of light, emits fluorescence in another wavelength. In addition to the original GFP, numerous variants that fluoresce in different colors, such as yellow (YFP), cyan (CFP), and blue (BFP), have been developed. We can use GFP and its variants as reporter genes on their own, or we can use them to generate fusion proteins in which a protein of interest is fused to GFP and can thus be visualized in living tissues, as is shown in the example in FIGURE 4.
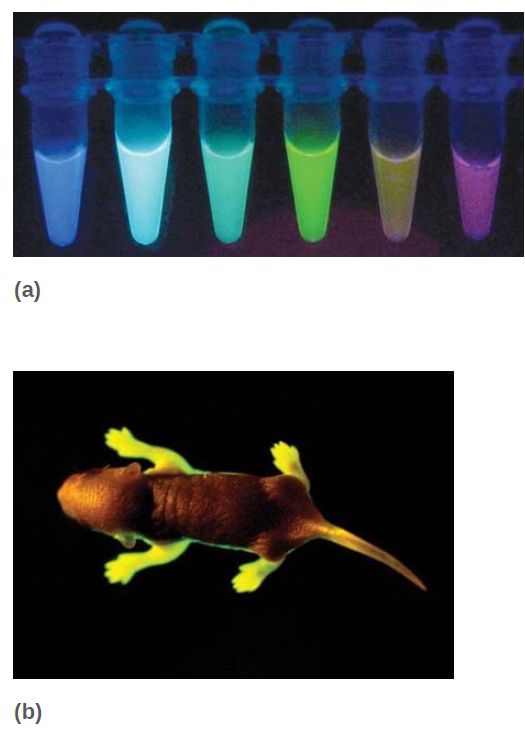
FIGURE 4 (a) Since the discovery of GFP, derivatives that fluoresce in different colors have been engineered. (b) A live transgenic mouse expressing human rhodopsin (a protein expressed in the retina of the eye) fused to GFP.
(a) Photo courtesy of Joachim Goedhart, Molecular Cytology, SILS, University of
Amsterdam. (b) © Eye of Science/Science Source.
Vectors are introduced into different species in a variety of ways. Bacteria and simple eukaryotes like yeast can be transformed easily, using chemical treatments that permeabilize the cell membranes . Many types of cells cannot be transformed so easily, though, and we must use other methods, as summarized in FIGURE 5. Some types of cloning vectors use natural methods of infection to pass the DNA into the cell, such as a viral vector that uses the viral infective process to enter the cell. Liposomes are small spheres made from artificial membranes, which can contain DNA or other biological materials. Liposomes can fuse with plasma membranes and release their contents into the cell. Microinjection uses a very fine needle to puncture the cell membrane. A solution containing DNA can be introduced into the cytoplasm or directly into the nucleus for cases in which the nucleus is large enough to be chosen as a target (such as an egg). The thick cell walls of plants are an impediment to many transfer methods; thus, the “gene gun” was invented as a means to overcome this obstacle. A gene gun shoots very small particles into the cell by propelling them through the wall at high velocity. The particles can consist of gold or nanospheres coated with DNA. This method now has been adapted for use with a variety of species, including mammalian cells.

FIGURE 5. DNA can be released into target cells by methods that pass it across the membrane naturally, such as by means of a viral vector (in the same way as a viral infection) or by encapsulating it in a liposome (which fuses with the membrane).
Alternatively, it can be passed manually, by microinjection, or by coating it on the exterior of nanoparticles that are shot into the cell by a “gene gun” that punctures the membrane at very high velocity.



|
|
|
|
دراسة يابانية لتقليل مخاطر أمراض المواليد منخفضي الوزن
|
|
|
|
|
|
|
اكتشاف أكبر مرجان في العالم قبالة سواحل جزر سليمان
|
|
|
|
|
|
|
اتحاد كليات الطب الملكية البريطانية يشيد بالمستوى العلمي لطلبة جامعة العميد وبيئتها التعليمية
|
|
|