


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 5-10-2020
Date: 2-10-2017
Date: 2-10-2017
|
As we begin to look at organic redox reactions, it is useful to consider how we define the oxidation state for carbon. Most of the redox reactions in this chapter involve a change in the oxidation state of the carbon bearing the functional group. To calculate the oxidation state for carbon, use the following guidelines:
So unlike metals, which are almost always in a positive oxidation state, the oxidation state of carbon can vary widely, from -4 (in CH4) to +4 (such as in CO2). Here are some examples.
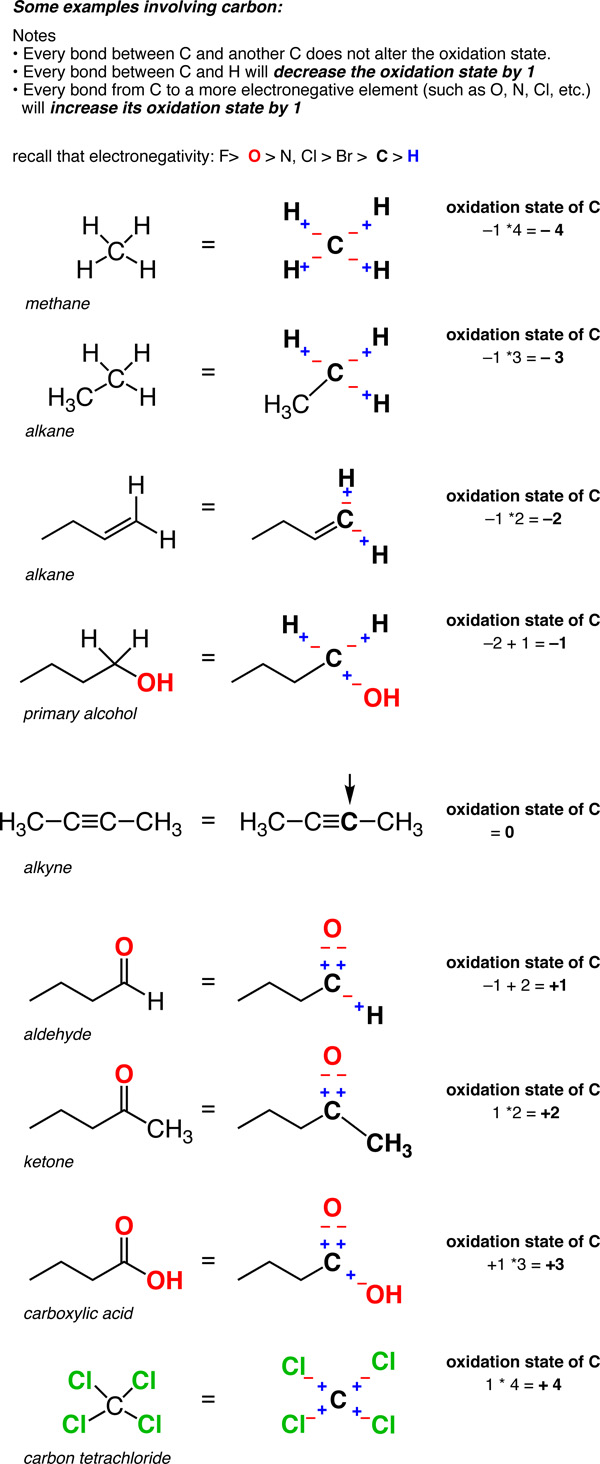
(Don’t forget that this is called a “formalism” for a reason. The charge on the carbon is not really +4 or –4. But the oxidation state formalism helps us keep track of where the electrons are going, which will come in handy very soon).
With an understanding of how to calculate oxidation states on carbon, we’re ready for the next step: understanding changes in the oxidation state at carbon, through reactions known as oxidations (where the oxidation state is increased), and reductions (where the oxidation state is reduced). More on that next time.



|
|
|
|
دراسة يابانية لتقليل مخاطر أمراض المواليد منخفضي الوزن
|
|
|
|
|
|
|
اكتشاف أكبر مرجان في العالم قبالة سواحل جزر سليمان
|
|
|
|
|
|
|
اتحاد كليات الطب الملكية البريطانية يشيد بالمستوى العلمي لطلبة جامعة العميد وبيئتها التعليمية
|
|
|