


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 31-5-2016
Date: 4-9-2019
Date: 27-11-2019
|
Preparation
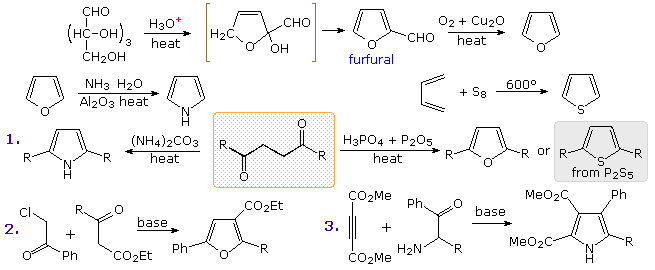
Commercial preparation of furan proceeds by way of the aldehyde, furfural, which in turn is generated from pentose containing raw materials like corncobs, as shown in the uppermost equation below. Similar preparations of pyrrole and thiophene are depicted in the second row equations. Equation 1 in the third row illustrates a general preparation of substituted furans, pyrroles and thiophenes from 1,4-dicarbonyl compounds, known as the Paal-Knorr synthesis. Many other procedures leading to substituted heterocycles of this kind have been devised. Two of these are shown in reactions 2 and 3. Furan is reduced to tetrahydrofuran by palladium-catalyzed hydrogenation. This cyclic ether is not only a valuable solvent, but it is readily converted to 1,4-dihalobutanes or 4-haloalkylsulfonates, which may be used to prepare pyrrolidine and thiolane.
Dipolar cycloaddition reactions often lead to more complex five-membered heterocycles.
Indole is probably the most important fused ring heterocycle in this class. By clicking on the above diagram three examples of indole synthesis will be displayed. The first proceeds by an electrophilic substitution of a nitrogen-activated benzene ring. The second presumably takes place by formation of a dianionic species in which the ArCH2(–) unit bonds to the deactivated carbonyl group. Finally, the Fischer indole synthesis is a remarkable sequence of tautomerism, sigmatropic rearrangement, nucleophilic addition, and elimination reactions occurring subsequent to phenylhydrazone formation. This interesting transformation involves the oxidation of two carbon atoms and the reduction of one carbon and both nitrogen atoms.
Reactions
The chemical reactivity of the saturated members of this class of heterocycles: tetrahydrofuran, thiolane and pyrrolidine, resemble that of acyclic ethers, sulfides, and 2º-amines, and will not be described here. 1,3-Dioxolanes and dithiolanes are cyclic acetals and thioacetals. These units are commonly used as protective groups for aldehydes and ketones, and may be hydrolyzed by the action of aqueous acid.
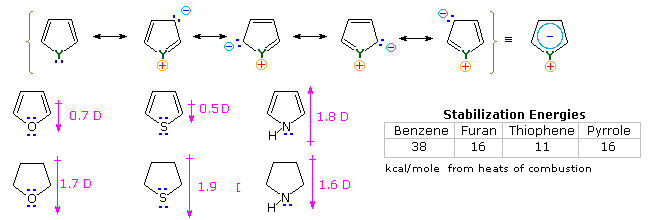
It is the "aromatic" unsaturated compounds, furan, thiophene and pyrrole that require our attention. In each case the heteroatom has at least one pair of non-bonding electrons that may combine with the four π-electrons of the double bonds to produce an annulene having an aromatic sextet of electrons. This is illustrated by the resonance description at the top of the following diagram. The heteroatom Y becomes sp2-hybridized and acquires a positive charge as its electron pair is delocalized around the ring. An easily observed consequence of this delocalization is a change in dipole moment compared with the analogous saturated heterocycles, which all have strong dipoles with the heteroatom at the negative end. As expected, the aromatic heterocycles have much smaller dipole moments, or in the case of pyrrole a large dipole in the opposite direction. An important characteristic of aromaticity is enhanced thermodynamic stability, and this is usually demonstrated by relative heats of hydrogenation or heats of combustion measurements. By this standard, the three aromatic heterocycles under examination are stabilized, but to a lesser degree than benzene.
Additional evidence for the aromatic character of pyrrole is found in its exceptionally weak basicity (pKa ca. 0) and strong acidity (pKa = 15) for a 2º-amine. The corresponding values for the saturated amine pyrrolidine are: basicity 11.2 and acidity 32.
Another characteristic of aromatic systems, of particular importance to chemists, is their pattern of reactivity with electrophilic reagents. Whereas simple cycloalkenes generally give addition reactions, aromatic compounds tend to react by substitution. As noted for benzene and its derivatives, these substitutions take place by an initial electrophile addition, followed by a proton loss from the "onium" intermediate to regenerate the aromatic ring. The aromatic five-membered heterocycles all undergo electrophilic substitution, with a general reactivity order: pyrrole >> furan > thiophene > benzene. Some examples are given in the following diagram. The reaction conditions show clearly the greater reactivity of furan compared with thiophene. All these aromatic heterocycles react vigorously with chlorine and bromine, often forming polyhalogenated products together with polymers. The exceptional reactivity of pyrrole is evidenced by its reaction with iodine (bottom left equation), and formation of 2-acetylpyrrole by simply warming it with acetic anhydride (no catalyst).
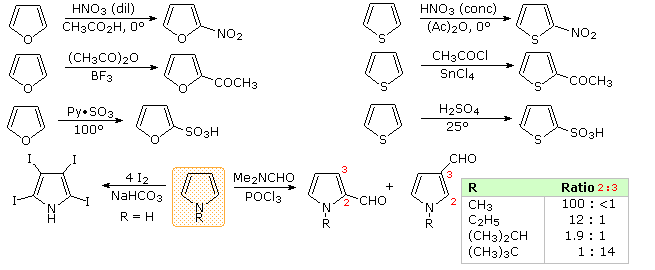
There is a clear preference for substitution at the 2-position (α) of the ring, especially for furan and thiophene. Reactions of pyrrole require careful evaluation, since N-protonation destroys its aromatic character. Indeed, N-substitution of this 2º-amine is often carried out prior to subsequent reactions. For example, pyrrole reacts with acetic anhydride or acetyl chloride and triethyl amine to give N-acetylpyrrole. Consequently, the regioselectivity of pyrrole substitution is variable, as noted by the bottom right equation.
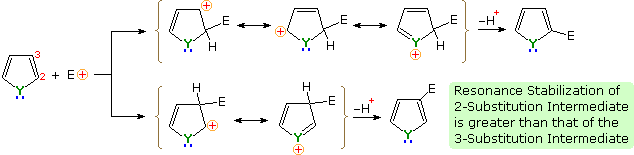
An explanation for the general α-selectivity of these substitution reactions is apparent from the mechanism outlined below. The intermediate formed by electrophile attack at C-2 is stabilized by charge delocalization to a greater degree than the intermediate from C-3 attack. From the Hammond postulate we may then infer that the activation energy for substitution at the former position is less than the latter substitution.
Functional substituents influence the substitution reactions of these heterocycles in much the same fashion as they do for benzene. Indeed, once one understands the ortho-para and meta-directing character of these substituents, their directing influence on heterocyclic ring substitution is not difficult to predict. The following diagram shows seven such reactions. Reactions 1 & 2 are 3-substituted thiophenes, the first by an electron donating substituent and the second by an electron withdrawing group. The third reaction has two substituents of different types in the 2 and 5-positions. Finally, examples 4 through 7 illustrate reactions of 1,2- and 1,3-oxazole, thiazole and diazole. Note that the basicity of the sp2-hybridized nitrogen in the diazoles is over a million times greater than that of the apparent sp3-hybridized nitrogen, the electron pair of which is part of the aromatic electron sextet.
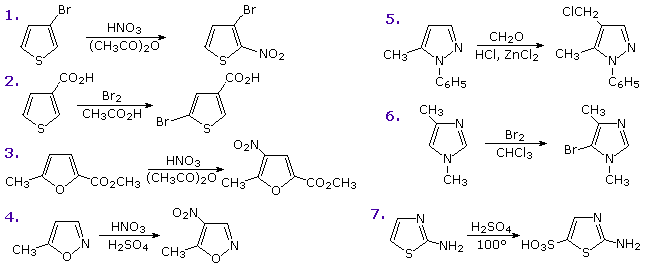
Other possible reactions are suggested by the structural features of these heterocycles. For example, furan could be considered an enol ether and pyrrole an enamine. Such functions are known to undergo acid-catalyzed hydrolysis to carbonyl compounds and alcohols or amines. Since these compounds are also heteroatom substituted dienes, we might anticipate Diels-Alder cycloaddition reactions with appropriate dienophiles. These possibilities will be illustrated above by clicking on the diagram. As noted in the upper example, furans may indeed be hydrolyzed to 1,4-dicarbonyl compounds, but pyrroles and thiophenes behave differently. The second two examples, shown in the middle, demonstrate typical reactions of furan and pyrrole with the strong dienophile maleic anhydride. The former participates in a cycloaddition reaction; however, the pyrrole simply undergoes electrophilic substitution at C-2. Thiophene does not easily react with this dienophile.
The bottom line of the new diagram illustrates the remarkable influence that additional nitrogen units have on the hydrolysis of a series of N-acetylazoles in water at 25 ºC and pH=7. The pyrrole compound on the left is essentially unreactive, as expected for an amide, but additional nitrogens markedly increase the rate of hydrolysis. This effect has been put to practical use in applications of the acylation reagent 1,1'-carbonyldiimidazole (Staab's reagent).
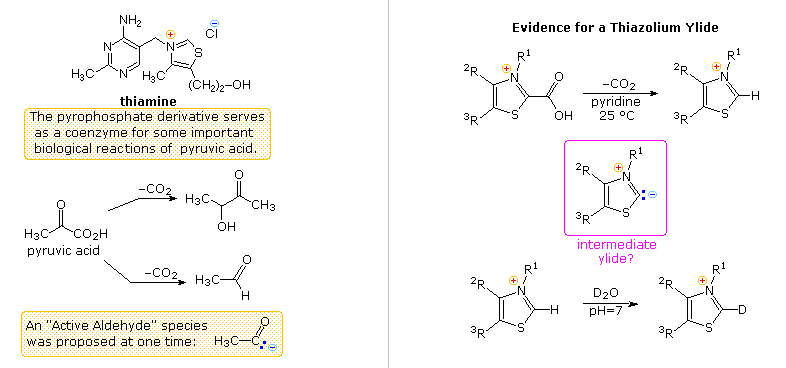
Another facet of heterocyclic chemistry was disclosed in the course of investigations concerning the action of thiamine (following diagram). As its pyrophosphate derivative, thiamine is a coenzyme for several biochemical reactions, notably decarboxylations of pyruvic acid to acetaldehyde and acetoin. Early workers speculated that an "active aldehyde" or acyl carbanion species was an intermediate in these reactions. Many proposals were made, some involving the aminopyrimidine moiety, and others, ring-opened hydrolysis derivatives of the thiazole ring, but none were satisfactory. This puzzle was solved when R. Breslow (Columbia) found that the C-2 hydrogen of thiazolium salts was unexpectedly acidic (pKa ca. 13), forming a relatively stable ylide conjugate base. As shown, this rationalizes the facile decarboxylation of thiazolium-2-carboxylic acids and deuterium exchange at C-2 in neutral heavy water.
Appropriate thiazolium salts catalyze the conversion of aldehydes to acyloins in much the same way that cyanide ion catalyzes the formation of benzoin from benzaldehyde, the benzoin condensation. By clicking on the diagram, a new display will show mechanisms for these two reactions. Note that in both cases an acyl anion equivalent is formed and then adds to a carbonyl function in the expected manner. The benzoin condensation is limited to aromatic aldehydes, but the use of thiazolium catalysts has proven broadly effective for aliphatic and aromatic aldehydes. This approach to acyloins employs milder conditions than the reduction of esters to enediol intermediates by the action of metallic sodium .
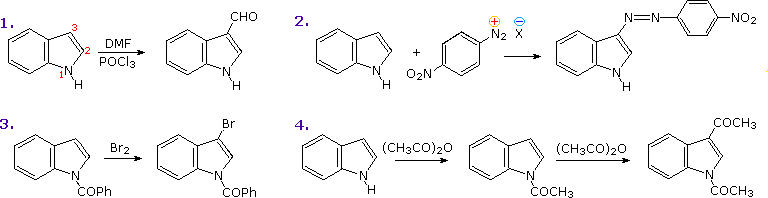
The most important condensed ring system related to these heterocycles is indole. Some electrophilic substitution reactions of indole are shown in the following diagram. Whether the indole nitrogen is substituted or not, the favored site of attack is C-3 of the heterocyclic ring. Bonding of the electrophile at that position permits stabilization of the onium-intermediate by the nitrogen without disruption of the benzene aromaticity.
Reference
http://www2.chemistry.msu.edu/faculty/reusch/VirtTxtJml/heterocy.htm#top1



|
|
|
|
أكبر مسؤول طبي بريطاني: لهذا السبب يعيش الأطفال حياة أقصر
|
|
|
|
|
|
|
طريقة مبتكرة لمكافحة الفيروسات المهددة للبشرية
|
|
|
|
|
|
|
قسم العلاقات العامة يناقش مع تربية بابل إطلاق الجدار الثقافي لمدارسها
|
|
|