


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 5-9-2017
Date: 19-1-2016
Date: 19-1-2016
|
Cracking Catalysts
Acid-treated clays were the first catalysts used in catalytic cracking processes, but have been replaced by synthetic amorphous silica-alumina, which is more active and stable. Incorporating zeolites (crystalline alumina- silica) with the silica/alumina catalyst improves selectivity towards aromatics. These catalysts have both Lewis and Bronsted acid sites that promote carbonium ion formation. An important structural feature of zeolites is the presence of holes in the crystal lattice, which are formed by the silica-alumina tetrahedra. Each tetrahedron is made of four oxygen anions with either an aluminum or a silicon cation in the center. Each oxygen anion with a –2 oxidation state is shared between either two silicon, two aluminum, or an aluminum and a silicon cation.
The four oxygen anions in the tetrahedron are balanced by the +4 oxidation state of the silicon cation, while the four oxygen anions connecting the aluminum cation are not balanced. This results in –1 net charge, which should be balanced. Metal cations such as Na+, Mg2+, or protons (H+) balance the charge of the alumina tetrahedra. A two dimensional representation of an H-zeolite tetrahedra is shown:
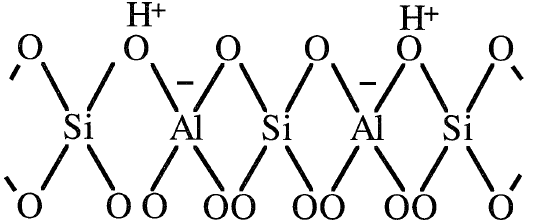
Bronsted acid sites in HY-zeolites mainly originate from protons that neutralize the alumina tetrahedra. When HY-zeolite (X- and Y-zeolites are cracking catalysts ) is heated to temperatures in the range of 400–500°C, Lewis acid sites are formed.

Zeolites as cracking catalysts are characterized by higher activity and better selectivity toward middle distillates than amorphous silica-alumina catalysts. This is attributed to a greater acid sites density and a higher adsorption power for the reactants on the catalyst surface.
The higher selectivity of zeolites is attributed to its smaller pores, which allow diffusion of only smaller molecules through their pores, and to the higher rate of hydrogen transfer reactions. However, the silicaalumina matrix has the ability to crack larger molecules. Hayward and Winkler have recently demonstrated the importance of the interaction of the zeolite with the silica-alumina matrix. In a set of experiments using gas oil and rare earth zeolite/silica-alumina, the yield of gasoline increased when the matrix was used before the zeolite. This was explained by the mechanism of initial matrix cracking of large feedstock
molecules to smaller ones and subsequent zeolite cracking of the smaller molecules to products. Aluminum distribution in zeolites is also important to the catalytic activity. An inbalance in charge between the silicon atoms in the zeolite framework creates active sites, which determine the predominant reactivity and selectivity of FCC catalyst. Selectivity and octane performance are correlated with unit cell size, which in turn can be correlated with the number of aluminum atoms in the zeolite framework.
Deactivation of zeolite catalysts occurs due to coke formation and to poisoning by heavy metals. In general, there are two types of catalyst deactivation that occur in a FCC system, reversible and irreversible. Reversible deactivation occurs due to coke deposition. This is reversed by burning coke in the regenerator. Irreversible deactivation results as a combination of four separate but interrelated mechanisms: zeolite dealumination, zeolite decomposition, matrix surface collapse, and contamination by metals such as vanadium and sodium.
Pretreating the feedstocks with hydrogen is not always effective in reducing heavy metals, and it is expensive. Other means that proved successful are modifying the composition and the microporous structure of the catalyst or adding metals like Sb, Bi or Sn, or Sb-Sn combination. Antimony organics have been shown to reduce by 50% gas formation due to metal contaminants, especially nickel.



|
|
|
|
دراسة يابانية لتقليل مخاطر أمراض المواليد منخفضي الوزن
|
|
|
|
|
|
|
اكتشاف أكبر مرجان في العالم قبالة سواحل جزر سليمان
|
|
|
|
|
|
|
المجمع العلمي ينظّم ندوة حوارية حول مفهوم العولمة الرقمية في بابل
|
|
|