


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 15-9-2020
Date: 23-1-2022
Date: 7-7-2019
|
Electrophilic Addition Reactions of Alkenes
Before beginning a detailed discussion of alkene reactions. Alkenes behave as nucleophiles (Lewis bases) in polar reactions, donating a pair of electrons from their electron-rich C=C bond to an electrophile (Lewis acid). For example, reaction of 2-methylpropene with HBr yields 2-bromo- 2-methylpropane. A careful study of this and similar reactions by Christopher Ingold and others in the 1930s led to the generally accepted mechanism shown in Figure 1.1 for electrophilic addition reactions.
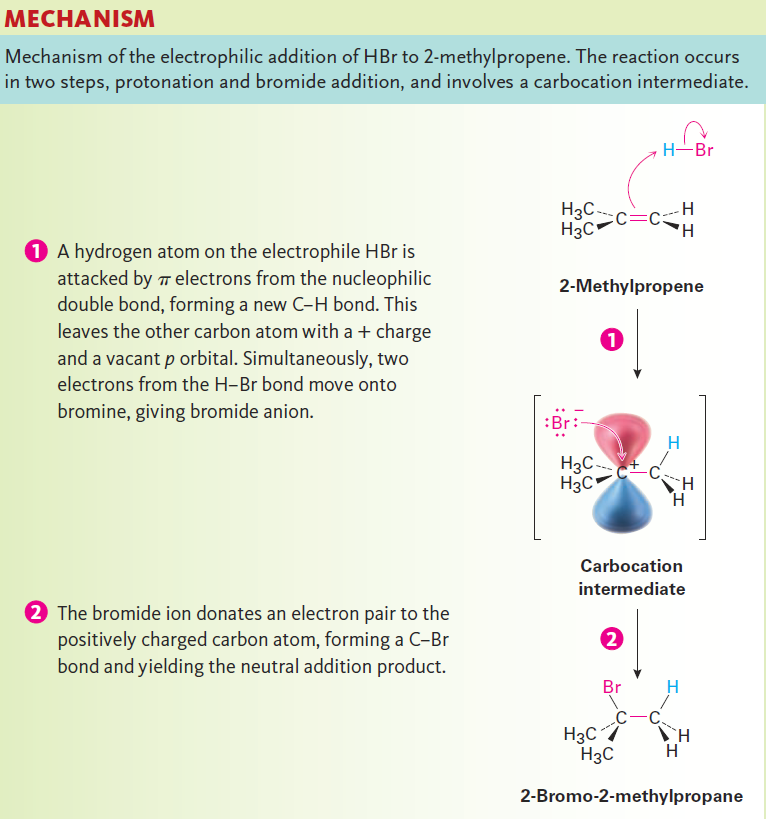
Figure 1.1
The reaction begins with an attack on the hydrogen of the electrophile HBr by the electrons of the nucleophilic π bond. Two electrons from the π bond form a new s bond between the entering hydrogen and an alkene carbon, as shown by the curved arrow at the top of Figure 1.1. The resulting carbocation intermediate is itself an electrophile, which can accept an electron pair from nucleophilic Br2 ion to form a C - Br bond and yield a neutral addition product.
An energy diagram for the overall electrophilic addition reaction (Figure 1.2) has two peaks (transition states) separated by a valley (carbocation intermediate). The energy level of the intermediate is higher than that of the starting alkene, but the reaction as a whole is exergonic (negative ΔG°). The first step, protonation of the alkene to yield the intermediate cation, is relatively slow. But once the cation intermediate is formed, it rapidly reacts to yield the final alkyl bromide product. The relative rates of the two steps are indicated in Figure 1.2 by the fact that ΔG1‡ is larger than ΔG2‡.
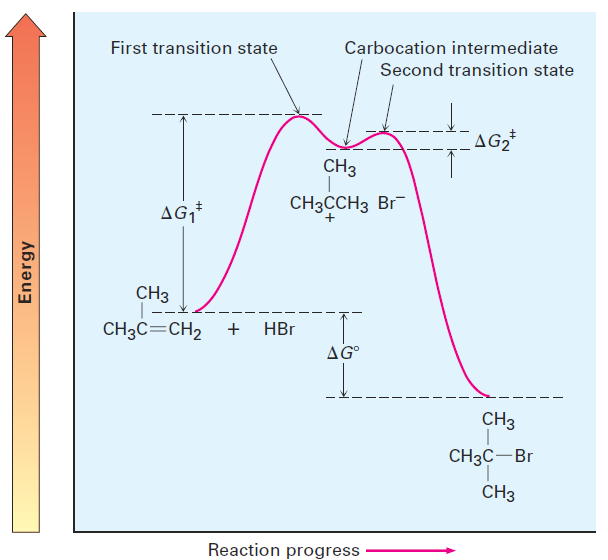
Figure 1.2 Energy diagram for the two-step electrophilic addition of HBr to 2-methylpropene. The first step is slower than the second step.
Electrophilic addition to alkenes is successful not only with HBr but with HCl, HI, and H2O as well. Note that HI is usually generated in the reaction mixture by treating potassium iodide with phosphoric acid and that a strong acid catalyst is needed for the addition of water.
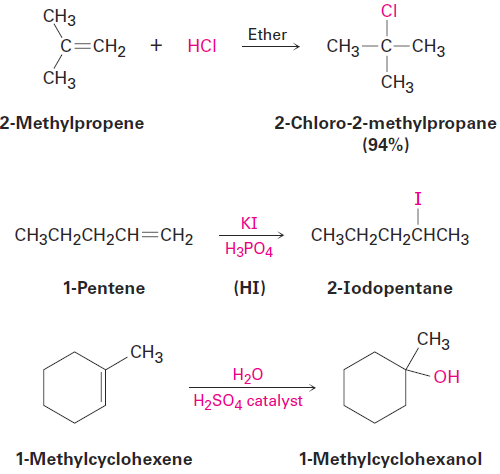



|
|
|
|
تفوقت في الاختبار على الجميع.. فاكهة "خارقة" في عالم التغذية
|
|
|
|
|
|
|
أمين عام أوبك: النفط الخام والغاز الطبيعي "هبة من الله"
|
|
|
|
|
|
|
قسم شؤون المعارف ينظم دورة عن آليات عمل الفهارس الفنية للموسوعات والكتب لملاكاته
|
|
|