


 الفيزياء الكلاسيكية
الفيزياء الكلاسيكية
 الكهربائية والمغناطيسية
الكهربائية والمغناطيسية
 علم البصريات
علم البصريات
 الفيزياء الحديثة
الفيزياء الحديثة
 النظرية النسبية
النظرية النسبية
 الفيزياء النووية
الفيزياء النووية
 فيزياء الحالة الصلبة
فيزياء الحالة الصلبة
 الليزر
الليزر
 علم الفلك
علم الفلك
 المجموعة الشمسية
المجموعة الشمسية
 الطاقة البديلة
الطاقة البديلة
 الفيزياء والعلوم الأخرى
الفيزياء والعلوم الأخرى
 مواضيع عامة في الفيزياء
مواضيع عامة في الفيزياء|
Read More
Date: 26-8-2016
Date: 21-8-2016
Date: 28-7-2016
|
Modified Joule–Thomson
Figure 1.1 shows container A of variable volume V controlled by a frictionless piston, immersed in a bath at temperature τ. This container is connected by a pipe with a porous plug to another container, B, of fixed volume V'. Container A is initially occupied by an ideal gas at pressure P while container B is initially evacuated. The gas is allowed to flow through the plug, and the pressure on the piston is maintained at the constant value P. When the pressure of the gas in B reaches P, the experiment is terminated. Neglecting any heat conduction through the plug, show that the final temperature of the gas in B is τ1 = (CP/CV) τ, where Cp and CV are the molar heats at constant pressure and volume of the gas.
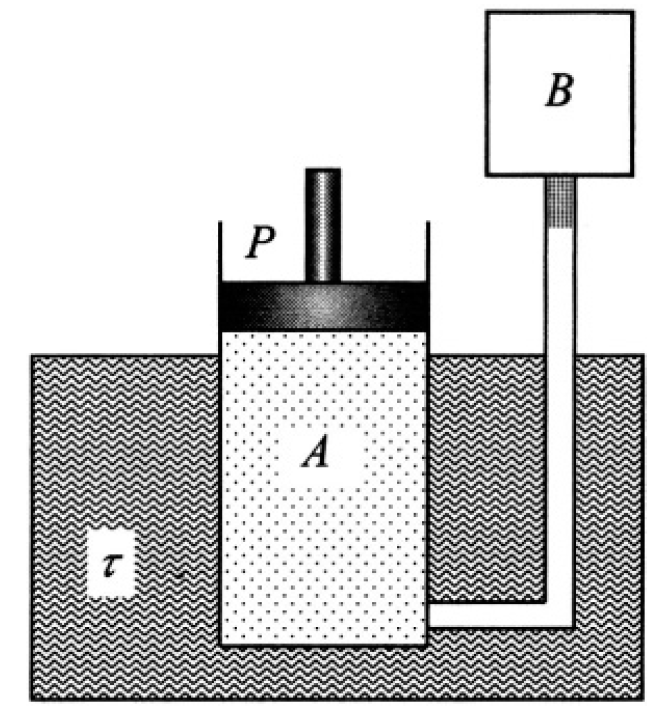
Figure 1.1
SOLUTION
The work done by the piston goes into changing the internal energy of the part of the gas of volume dV that enters the plug and into the work done by the gas to enter container B occupying volume dV'. So we may write
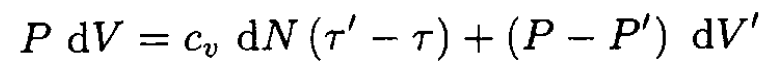 (1)
(1)
where cv is the constant-volume heat capacity for one molecule and dN is the number of molecules in the volume dV. On the other hand, before and after the plug, we have, respectively,
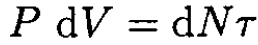 (2)
(2)
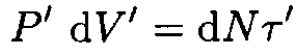 (3)
(3)
Substituting dV from (2) and from (3) into (1), we have
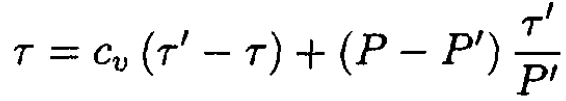 (4)
(4)
So,
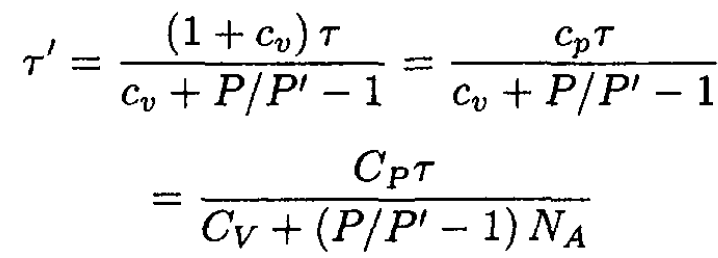 (5)
(5)
When P = P' (5) becomes
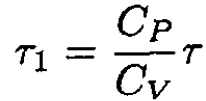 (6)
(6)



|
|
|
|
تفوقت في الاختبار على الجميع.. فاكهة "خارقة" في عالم التغذية
|
|
|
|
|
|
|
أمين عام أوبك: النفط الخام والغاز الطبيعي "هبة من الله"
|
|
|
|
|
|
|
قسم شؤون المعارف ينظم دورة عن آليات عمل الفهارس الفنية للموسوعات والكتب لملاكاته
|
|
|