


 النبات
النبات
 الحيوان
الحيوان
 الأحياء المجهرية
الأحياء المجهرية
 علم الأمراض
علم الأمراض
 التقانة الإحيائية
التقانة الإحيائية
 التقنية الحيوية المكروبية
التقنية الحيوية المكروبية
 التقنية الحياتية النانوية
التقنية الحياتية النانوية
 علم الأجنة
علم الأجنة
 الأحياء الجزيئي
الأحياء الجزيئي
 علم وظائف الأعضاء
علم وظائف الأعضاء
 الغدد
الغدد
 المضادات الحيوية
المضادات الحيوية|
Read More
Date: 9-5-2021
Date: 24-3-2021
Date: 16-5-2016
|
Glutathione
Glutathione was first discovered by J. de Rey-Pailhade over 100 years ago, and its structure (ie, L-g-glutamyl-L-cysteinyl-glycine, or g-Glu-Cys-Gly),
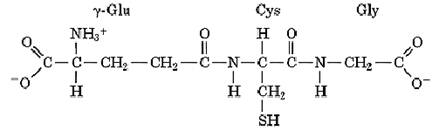
was deduced in the 1930s. It has several important functions: (i) most importantly, as a reductant; (ii( conjugation to foreign chemicals to make them more water-soluble, less toxic, and excretable; (iii( transport of amino acids across cell membranes; (iv) incorporation into some leukotriene structures; (v) as a cofactor for some enzymes; (vi) as a nontoxic storage form of cysteine; and (vii) in forming and maintaining protein disulfide bonds in the endoplasmic reticulum (ER).
Glutathione is the predominant thiol compound in very many cells, both prokaryotes and eukaryotes, where its total intracellular concentration is usually in the range 0.5 to 12 mM. It is present in most eukaryotes, except for those that do not have mitochondria. It is not present in many Archaebacteria, but in halobacteria it is replaced by g-Glu-Cys. Likewise, some eubacteria do not have glutathione, and it is found primarily in the bacteria of the cyanobacteria and the purple bacteria classes. It is believed that glutathione arose initially in the prokaryotic ancestors of mitochondria and chloroplasts and was acquired by eukaryotes along with these organelles.
Glutathione is synthesized from its three constituent amino acids. First, the side-chain g-carboxyl group of glutamic acid is joined in a peptide bond to the a-amino group of cysteine, by the enzyme g-glutamylcysteine synthetase. The a-carboxyl group of the resulting g-Glu-Cys is then joined in a normal peptide bond with the a-amino group of glycine, catalyzed by glutathione synthetase. Both steps are coupled to the hydrolysis of ATP. Some species of plants have a variant of glutathione,
homoglutathione, in which the glycine residue is replaced by b-alanine, due to an evolutionary change in specificity of the enzyme glutathione synthetase. In trypanosomes, glutathione is largely replaced by the related trypanothione in which two g-Glu-Cys-Gly moieties are linked by peptide bonds through their carboxyl groups to a molecule of spermidine.
Glutathione is also degraded to its constituent amino acids in vivo, which is believed to give it an important role as a nontoxic storage form of cysteine. High levels of cysteine are toxic in some systems, in part because its amino, carboxyl, and thiol groups are well situated stereochemically to chelate metal ions, which catalyze air oxidation of its thiol group. A by-product of thiol oxidation is peroxides, which damage the cell. Glutathione is much more resistant to air oxidation and much less toxic, and it can occur safely at 10 to 100 times greater levels.
Glutathione is an essential cofactor for a number of enzymes, including formaldehyde dehydrogenase, glyoxylase, maleylacetoacetate isomerase, dehydrochlorinase, and prostaglandin endoperoxidase isomerase. The glutathione is involved transiently in the reactions they catalyze. For example, formaldehyde dehydrogenase uses NAD+ to catalyze the oxidation of an adduct of formaldehyde and glutathione to produce S-formylglutathione, which is subsequently hydrolyzed by a specific lyase to yield formate and regenerate the glutathione. Glyoxylase is similar, also generating a transient intermediate adduct between its substrate, methyl glyoxal, and glutathione.
Naturally, glutathione is found in a mixture of the thiol form, GSH, and with a disulfide bond linking two glutathione moieties, GSSG. The GSH thiol group has a pKa value of about 8.8, while the amino and carboxyl groups have normal pKa values that are virtually the same in the disulfide form (1). There apparently are no substantial interactions between the two glutathione moieties in GSSG, and the stability of this disulfide bond is comparable to that between two other cysteine residues on unfolded peptides or proteins; the rate and equilibrium constants for thiol–disulfide exchange are close to their expected values (2).
Within the cytosol of cells, glutathione is kept primarily in the reduced GSH form, largely by reduction of GSSG by the enzyme glutathione reductase, at the expense of NADPH:

The mechanism of this reaction is complex, as the enzyme contains a pair of cysteine residues that reversibly form a disulfide bond, plus one FAD molecule (3). As GSH and GSSG will react spontaneously with each other and with other thiol and disulfide compounds of the cell, in the thiol–disulfide exchange reaction, the ratio of GSH and GSSG reflects the general redox properties of the cell. Being present at such high concentrations, GSH is an important reductant within cells. For example, it is involved in the reduction of glutaredoxin, and it and NADPH reduce the oxidized form of vitamin C, dehydroascorbate, to the active form, ascorbate.
GSH is used for the destruction of oxidants, such as organic peroxides and H2O2, which would cause irreversible damage to membranes, DNA, and numerous other cellular components; this reaction is catalyzed by the enzyme glutathione peroxidase:

This enzyme is unusual in that it has a selenocysteine residue at its active site (4).
Normally, GSH predominates over GSSG by a factor of 100 in the cytosol. This is a somewhat reducing environment that tends to keep protein cysteine residues in the thiol form (HPSSH) and destabilizes any disulfide bonds they may make (PSS),

although very stable disulfide bonds found in some proteins could be present under these conditions. The stability of a protein disulfide bond is determined by its intrinsic stability, Keq, plus the ratio of the concentrations of GSH and GSSG:

Only very stable protein disulfide bonds, with large values of Keq, could be populated in the cytosol.
In the endoplasmic reticulum (ER) of eukaryotes, in contrast, GSH and GSSG are present in nearly equal concentrations (5). With 1 mM each of GSH and GSSG, the same disulfide bond would be present in the ER to an extent 100 times greater than in the cytosol. This is sufficient to permit proteins in the ER to form disulfide bonds if their conformations stabilize them; if not, the protein cysteine residues will tend to remain in the thiol form. The formation of such stable disulfide bonds in proteins in the ER is catalyzed by protein disulfide isomerase (PDI). PDI can have an unstable disulfide bond at each of its two active sites, which is readily transferred to other proteins. The PDI active-site disulfide bonds are believed to be regenerated by chemical reaction of the protein with GSSG.
Glutathione is believed to be used in some cells, such as those of the mammalian liver and kidney, for the transport of amino acids (except for proline) across the plasma membrane (Fig. 1). The extracellular amino acid is reacted with intracellular glutathione that has been shuttled to the cell surface by the integral membrane protein g-glutamyltranspeptidase. This replaces the Cys-Gly dipeptide of GSH by the amino acid and produces g-Glu-amino acid. The amino acid is liberated upon degradation of the g-Glu-amino acid by the enzyme g-glutamyl cyclotransferase, which generates 5-oxoproline instead of glutamate. The driving force for uptake of amino acids by this “group translocation” type of mechanism is the destruction of the glutathione, for the Cys-Gly moiety is also degraded, by cysteinylglycine dipeptidase; regeneration of glutamate from 5-oxoproline by 5-oxoprolinase requires hydrolysis of ATP, and resynthesis of glutathione requires another two molecules of ATP. The disulfide form of cysteine, cystine, is an especially reactive amino acid substrate of this cycle. The g-Glu-CysSSCys generated by g-glutamyltranspeptidase is rapidly reduced in the cell cytosol to g-Glu-Cys and cysteine. The g-Glu-Cys can then be converted to GSH by addition of glycine, thereby bypassing the production of 5-oxoproline. On the other hand, humans lacking g-glutamyltranspeptidase have no difficulty in transporting amino acids, and there are many other types of amino acid transporters. The cycle is now believed to play only a minor role in transport of amino acids, except for cysteine.
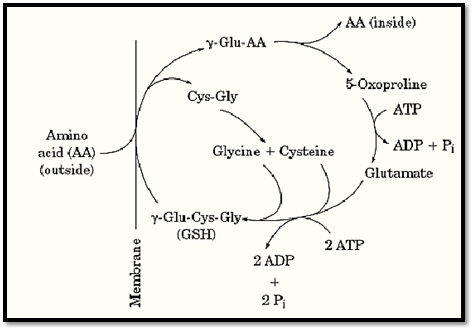
Figure 1. The g-glutamyl cycle for transporting amino acids, coupled to the degradation of glutathione (GSH; g-Glu-Cys-Gly). The amino acid (AA) on the outside of the cell (left) is coupled to glutamic acid of GSH by the membrane protein g-glutamyl transpeptidase, by replacing the dipeptide cysteinyl-glycine (Cys-Gly). The amino acid is released from the g-glutamyl residue, converting it to 5-oxoproline. The concomitant degradation of GSH is the driving force for transporting the amino acid. Resynthesizing 1 mol of GSH requires the hydrolysis of 3 moles of ATP.
The g-glutamyl cycle is now believed to have a major role in higher organisms in the metabolism of leukotrienes, estrogens, prostaglandins, and many drugs and xenobiotics. All these molecules, often after their reaction with cytochrome P-450, are linked covalently to the thiol group of GSH by the large enzyme family of glutathione S-transferases. The resulting glutathione S-conjugate is then degraded to the Cys-Gly S-conjugate and then to the cysteine S-conjugate by g-glutamyltranspeptidase and cysteinylglycine dipeptidase, respectively. The cysteine S-conjugate is converted, by a specific N-acetyltransferase to the corresponding mercapturate, N-acetylcysteine S-conjugate, which is then excreted. On the other hand, this pathway can also sometimes lead to the generation of a toxic substance. For example, halogenated cysteine S-conjugates can generate highly reactive thiol-containing fragments through the action of cysteine S-conjugate b-lyases:

where RSH is the thiol form of the xenobiotic, which can be toxic.
The generally protective, antioxidative effects of GSH are reflected in the observations that decreased levels of GSH lead to clinical symptoms in humans and decreased resistance to viruses and other diseases.
References
1. G. Jung, E. Beitmeier, and W. Voelter (1972) Eur. J. Biochem. 24, 438–445.
2. N. J. Darby and T. E. Creighton (1995) Biochemistry 34, 3576–3587.
3. P. A. Karplus and G. E. Schulz (1989) J. Mol. Biol. 210, 163–180.
4. O. Epp et al. (1983) Eur. J. Biochem. 133, 51–69.
5. C. Hwang, A. J. Sinskey, and H. F. Lodish (1992) Science 257, 1496–1502.



|
|
|
|
تفوقت في الاختبار على الجميع.. فاكهة "خارقة" في عالم التغذية
|
|
|
|
|
|
|
أمين عام أوبك: النفط الخام والغاز الطبيعي "هبة من الله"
|
|
|
|
|
|
|
قسم شؤون المعارف ينظم دورة عن آليات عمل الفهارس الفنية للموسوعات والكتب لملاكاته
|
|
|