


 النبات
النبات
 الحيوان
الحيوان
 الأحياء المجهرية
الأحياء المجهرية
 علم الأمراض
علم الأمراض
 التقانة الإحيائية
التقانة الإحيائية
 التقنية الحيوية المكروبية
التقنية الحيوية المكروبية
 التقنية الحياتية النانوية
التقنية الحياتية النانوية
 علم الأجنة
علم الأجنة
 الأحياء الجزيئي
الأحياء الجزيئي
 علم وظائف الأعضاء
علم وظائف الأعضاء
 الغدد
الغدد
 المضادات الحيوية
المضادات الحيوية|
Read More
Date: 10-11-2020
Date: 12-5-2016
Date: 23-2-2021
|
DNA-Dependent DNA Polymerases
DNA-dependent DNA polymerases synthesize deoxyribonucleic acid (DNA), a role that is central to accurately transmitting genetic material from generation to generation. This family of polymerases functions in a template-dependent manner to insert incoming nucleotides that are encoded by the template onto a growing primer. Polymerases are highly proficient at inserting nucleotides with proper Watson–Crick base pairing. DNA-dependent DNA polymerase exhibits unique roles during DNA replication and DNA repair. This article focuses on the characteristics of prokaryotic and eukaryotic DNA polymerases and the roles of each.
1. Primary Sequence, Structure, and Evolution
Sequence analysis studies (1) have divided the DNA-dependent DNA polymerase family into three subfamilies: (1) pol I type (which includes Taq polymerase and those from bacteriophage T5 and T7); (2) pol a type (which includes phage T4 polymerase and those from vaccinia, adenovirus, and herpes viruses); and (3) pol b type (which includes terminal deoxynucleotidyl transferase).
The presence of homologous regions in these diverse polymerases suggests that these enzymes evolved from a common ancestor. All three subfamilies share two common motifs (A and C), whereas only pol I and pol a types also share a third (B). The X-ray crystallographic structure of the Klenow fragment of pol I suggests that motif A is involved in binding the divalent metal cofactor and deoxynucleoside triphosphates (dNTP), motif B is involved in binding the template and bases of the incoming dNTP, and motif C is involved in binding the metal cofactor. The structure of pol b shows DNA bound in an orientation 180° opposite to that observed in the complexes of DNA with HIV-1 reverse transcriptase and with the Klenow fragment (2). Thus, it is thought the pol b and terminal deoxynucleotidyl transferase are evolutionarily distinct from the other DNA-dependent DNA polymerases (3).
2. Prokaryotic DNA Polymerases
Escherichia coli contain three polymerases, designated DNA pol I, II, and III.
2.1. Pol III
The holoenzyme of DNA pol III (Fig. 1) is composed of 10 polypeptide subunits and is present in approximately 15 copies per bacterial cell. It is the principle polymerase responsible for replicating the genome. Pol III is purified either intact or as a core enzyme separate from its accessory factors. The Pol III core consists of the polymerase subunit a (140 kDa; expressed from the DnaE gene; Ref. 4), a proofreading subunit (28 kDa; from dnaQ or mut D gene; Ref. 5), and subunit q (10 kDa; holE). The processivity (i.e., the number of nucleotides synthesized per binding event) of the core enzyme is about 10 (6). This processivity is enhanced to several thousand nucleotides by the addition of subunit b (40 kDa), which functions as a dimer resembling a hexagonal sliding clamp (7). The q subunit (71 kDa) dimerizes readily, which, it is thought, facilitates the interaction of two polymerase core molecules. Evidence suggests that two polymerase core molecules act in concert but in opposite directions to synthesize the leading and lagging strands simultaneously (Fig. 1). The final component of the pol III holoenzyme is the g complex, which is composed of two subunits each of d, d′, g, c, and j. The g complex is a DNA-dependent ATPase that functions as a clamp loader (8). The hydrolysis of ATP facilitates loading of subunit b onto double-stranded DNA.

Figure 1. Model of the pol III replication complex during leading and lagging strand synthesis. Dimerization of the domain facilitates the interaction of two core pol III molecules. This dimerization potentially allows concerted synthesis of both leading and lagging strands by one large polymerase complex. Other proteins that assist during replication in E. coli include DNA B helicase, primase, and single-stranded binding protein (SSB). Arrows show the 5′-3′ direction of polymerization.
2.2. Pol I
DNA polymerase I (pol I; expressed from the polA gene), is a single polypeptide chain that encodes at least three separate catalytic activities. It is present at approximately 400 copies per cell, functions during DNA repair in E. coli, and also exhibits a minor role in DNA replication. The three catalytic activities of pol I are: (1) 5′-3′ polymerization, (2) 3′-5′ exonuclease (proofreading); and (3) 5′-3′ exonuclease (involved in nick translation). Mild proteolysis of pol I yields an amino-terminal 5′-3′ exonuclease domain and the COOH-terminal Klenow fragment, which contains polymerization and 3′-5′ proofreading activities (9). For details on pol I functions see DNA Polymerase I and Klenow Fragment.
2.3. Pol II
DNA polymerase II (a product of the polB gene) contains a 3-5′ exonuclease activity, but lacks a 5′-3′ exonuclease. The amount of Pol II activity is comparable to that of pol III in crude extracts from PolA- E. coli, and its nucleotide sequence resembles pol a-type polymerases. However, the precise roles of pol II in DNA metabolism remain to be established. Mutational studies with E. coli show that the pol B gene is not required for growth or for repair after UV damage.
3. Eukaryotic Polymerases
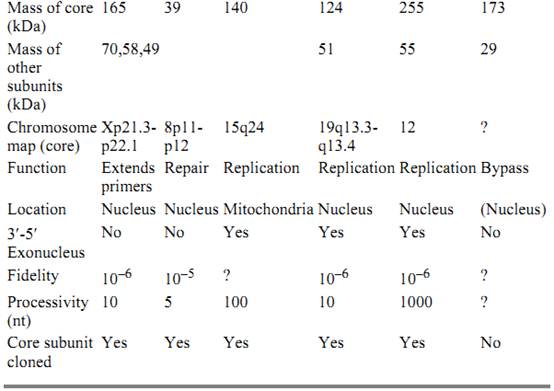
The six known polymerases in eukaryotes are pol a, b, g, d, Ɛ , and z (see Table 1). Much of our understanding of the proteins and their roles in eukaryotic replication fork has stemmed from studying the SV40 virus replication system in vitro (10).
Table 1. Eukaryotic Polymerases

3.1. DNA Polymerase a
This polymerase is composed of four subunits: (1) a 165- to 180-kDa phosphorylated glycoprotein that exhibits polymerase activity (its gene is located on human chromosome Xp21.3-p22.1.); (2) a 70-kDa phosphoprotein with unknown function; and (3 and 4) 49- and 58-kDa subunits that have primase activity (11). An early study in which monoclonal antibody against human DNA polymerase a was microinjected into mammalian cells showed that DNA replication is inhibited (12). Subsequent studies showed that this inhibition results from decreased extension of Okazaki Fragments. It was originally thought the primary function of pol a is to synthesize the lagging strand, but pol a's lack of an exonuclease domain and its poor processivity (about 10 nucleotides) cast some doubts. More recent studies of the SV40 replication system suggest that pol a functions to synthesize the RNA primer for both the leading and the lagging strands via its primase subdomain and to extend the RNA primer by several nucleotides on both strands via its polymerase domain (13). The majority of leading and lagging strand synthesis can potentially be carried out by polymerases d and Ɛ. Mutational analysis in yeast of active polymerase a, d, and Ɛ subunits show that each is essential for growth.
3.2. Polymerase b
This is a 39- to 45-kDa monomeric protein in vertebrates that is constitutively expressed (its gene is located on human chromosome 8p11-12) and considered the principle enzyme involved in filling in small DNA gaps resulting from excising damaged bases (14). Although expressed as a single polypeptide chain, pol b contains an N-terminal 8-kDa domain, which has apurinic lyase activity responsible for cleaving the 5′ phosphate-ribose residue during base excision repair, and a 31-kDa C-terminal domain that contains the polymerase active site. Pol b misincorporates noncomplementary nucleotides at a relatively high frequency, one per 5000 nucleotides polymerized (15) and lacks a 3′-5′ exonuclease (proofreading) activity. Thus, DNA repair by pol b may be highly error-prone. Pol b repairs gaps of six nucleotides or less highly processively but exhibits distributive synthesis in longer gaps. Of the three types of DNA repair, base excision repair (which leaves gaps of either one nucleotide or up to 10 nucleotides via unique mechanisms), nucleotide excision repair (which leaves gaps of about 30 nucleotides), and mismatch repair (which leaves gaps of hundreds of nucleotides,( it is thought that pol b is involved primarily during base excision repair. A knockout strain of the pol b gene is embryonically lethal in mice, indicating that pol b is essential for normal development (16) . However, the pol b-like gene of Saccharomyces cerevisiae (Pol4) may be disrupted without affecting yeast viability and only marginally affecting sensitivity to methylmethanesulfonate, a DNA alkylating agent. Apparently, either pol d or can substitute for pol b during base excision repair in S. cerevisiae (17). It has also been shown that the pol b localizes to synaptic foci during the prophase of meiosis I, suggesting that pol b activity is required to fill gaps during meiotic recombination in cells (18).
3.3. Polymerase g
This is a monomeric, 140-kDa enzyme that contains both polymerase and 3′-5′, exonuclease activities. Although encoded in the nucleus, pol g is primarily responsible for replicating and repairing the mitochondrial genome. The gene that encodes human pol g (located on chromosome 15q24) has just recently been cloned. The amino acid sequence of human pol g is 40 to 50% identical to pol g from yeast and Drosophila, pol g is also highly homologous to E. coli DNA pol I . (19)
3.4. Polymerase d
This polymerase consists of a large catalytic polypeptide chain of 124 kDa from a gene located on chromosome 19q13.3q13.4, and a small polypeptide chain of 51 kDa from a gene located on chromosome 7. Mammalian pol d was first discovered in and purified from rabbit bone marrow, the catalytic subunit of human DNA pol d has been cloned and expressed in Baculovirus-infected cells (20). It is one of two nuclear mammalian DNA polymerases that has 3′-5′ exonuclease ) proofreading) activity (21). The processivity of pol d is approximately 10 nucleotides, but this increases to well over 1000 nucleotides after the enzyme associates with a homotrimer of 36-kDa subunits of proliferating cellular nuclear antigen (PCNA), which forms a sliding clamp (22). In the SV40 replication system, pol d conducts most of both leading and lagging strand synthesis, complete replication in this system requires PCNA. Other proteins required for efficient pol d function include replication factor C (a phosphatase that loads PCNA onto the Pol d-DNA complex via an ATP-dependent reaction) and replication protein A (a single-stranded binding protein involved in reducing secondary structures of the template strand). Because of its high processivity, the Pol d–PCNA complex may also have important roles in filling DNA gaps during mismatch repair, nucleotide excision repair, and long-patch base excision repair (17).
3.5. PolymeraseƐ
This polymerase has a large catalytic subunit of 170 kDa, whose gene is located on chromosome 12q24.3. It shares many properties with pol d, including the presence of 3′-5′ exonuclease activity and very high processivity. Like pol d, pol Ɛ was also first discovered in rabbit bone marrow. Human pol Ɛ has been purified from HeLa cells and from placenta. The precise size of pol Ɛ remains controversial, as its various subunits continue to be identified. Interestingly, with multisubunit DNA polymerases like pol d and pol Ɛ the large subunit is invariably responsible for DNA polymerization. Unlike pol d, pol Ɛ is thought to be highly processive and synthesizes approximately 1000 nucleotides per binding event even when not associated with PCNA. Pol Ɛ interacts with PCNA at physiological ionic concentrations, thus further augmenting the enzyme's processivity and activity. The in vivo roles of pol d and Ɛ have not been adequately distinguished, but it is thought that both have roles in leading and lagging strand synthesis and in mismatch, nucleotide excision, and long-patch base excision repair.
3.6. Polymerase z
This sixth DNA polymerase has been described in S. cerevisiae. Pol z is composed of two subunits of 173 kDa (product of the yeast REV3 gene) and 29 kDa (REV7 gene). The Rev3–Rev7 complex incorporates nucleotides across damaged DNA templates (23). DNA lesions including thymine dimers, abasic sites, and DNA adducts are potent at arresting DNA synthesis. The translesion bypass function ascribed to pol z could be important when efficient and rapid DNA synthesis across damaged DNA is needed during replication.
References
1. M. Delarue, O. Poch, N. Tordo, D. Moras, and P. Argos (1990) Protein Eng. 3, 461–467.
2. H. Pelletier, M. R. Sawaya, A. Kumar, S. H. Wilson, and J. Kraut (1994) Science 264, 1891–1903.
3. P. H. Patel, A. Jacobo-Molina, J. Ding, C. Tantillo, A. D. Clark Jr., R. Raag, R. G. Nanni, S. H. Hughes, and E. Arnold (1995) Biochemistry 34, 5351–5363.
4. H. Maki and A. Kornberg (1985) J. Biol. Chem. 260, 12987–12982.
5. R. H. Scheuermann and H. Echols (1984) Proc. Natl. Acad. Sci. USA 81, 7747–7751.
6. P. J. Fay, K. O. Johanson, C. S. McHenry, and R. A. Bambera (1982) J. Biol. Chem. 257, 5692–5699.
7. P. T. Stukenberg, P. S. Studwell-Waughan, and M. O''Donnell (1991) J. Biol. Chem. 266, 11328-11334.
8. R. Onrust, P. T. Stukenberg, and M. O''Donnell (1991) J. Biol. Chem. 266, 21681–21686.
9. D. Brutlag and A. Kornberg (1972) J. Biol. Chem. 247, 241–248.
10. J. Li and T. J. Kelly (1984) Proc. Natl. Acad. Sci. USA 81, 6973–6977.
11. D. A. Adler, B. Y. Tseng, T. S. Wang, and C. M. Disteche (1991) Genomics 9, 642–646.
12. L. Kaczmarek, M. R. Miller, R. A. Hammond, and W. E. Mercer (1986) J. Biol. Chem. 261, 10802-10802.
13. R. J. Hickey and L. H. Malkas (1997) Crit. Rev. Eukaryotic. Gene Expression 7, 125–157.
14. J. Abbotts, D. N. SenGupta, B. Zmudzka, S. G. Widen, V. Notario, and S. H. Wilson (1988( Biochemistry 27, 901–909.
15. T. A. Kunkel and L. A. Loeb (1981) Science 213, 765–767.
16. H. Gu, J. D. Marth, P. C. Orban, H. Mossmann, and K. Rajewsky (1994) Science 265, 26–28.
17. A. Blank, B. Kim, and L. A. Loeb (1994) Proc. Natl. Acad. Sci. USA 91, 9067–9051.
18. A. W. Plug, C. A. Clairmont, E. Sapi, T. Ashley, and J. B. Sweasy (1997) Proc. Natl. Acad. Sci. USA 94, 1327–1331.
19. P. A. Ropp and W. C. Copeland (1996) Genomics 36, 449–458.
20. J. Q. Zhou, C. K. Tan, A. G. So, and K. M. Downey (1996) J. Biol. Chem. 271, 29740–29745.
21. J. J. Byrnes, K. M. Downey, B. G. Que, M. Y. Lee, V. L. Black, and A. G. So (1977( Biochemistry 16, 3740–3746.
22. T. S. Krishna, X. P. Kong, S. Gary, P. M. Burgers, and J. Kuriyan (1994) Cell 79, 1233–1243.
23. J. R. Nelson, C. W. Lawrence, and D. C. Hinkle (1996) Science 272, 1646–1649.



|
|
|
|
هل يمكن أن تكون الطماطم مفتاح الوقاية من السرطان؟
|
|
|
|
|
|
|
اكتشاف عرائس"غريبة" عمرها 2400 عام على قمة هرم بالسلفادور
|
|
|
|
|
|
|
جامعة الكفيل تقيم ندوة علمية عن الاعتماد الأكاديمي في جامعة جابر بن حيّان
|
|
|