
آخر المواضيع المضافة


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 13-9-2019
Date: 28-11-2019
Date: 28-7-2018
|
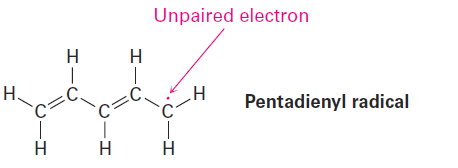
Drawing Resonance Forms for a Radical
Draw three resonance forms for the pentadienyl radical, where a radical is a substance that contains a single, unpaired electron in one of its orbitals, denoted by a dot ((..
S t r a t e g y
Find the three-atom groupings that contain a multiple bond next to an atom with a p orbital.
S o l u t i o n
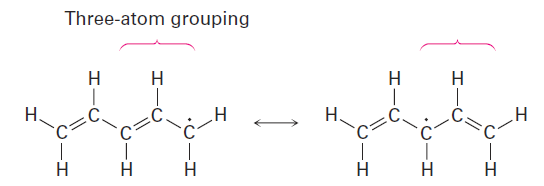
The unpaired electron is on a carbon atom next to a C5C bond, giving a typical three-atom grouping that has two resonance forms.
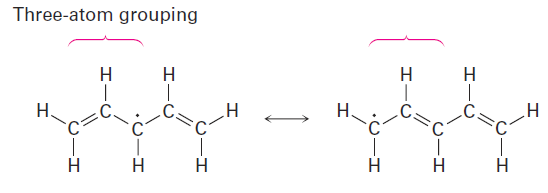
In the second resonance form, the unpaired electron is next to another double bond, giving another three-atom grouping and leading to another resonance form.
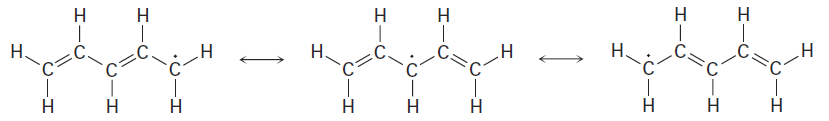
Thus, the three resonance forms for the pentadienyl radical are:



|
|
|
|
تفوقت في الاختبار على الجميع.. فاكهة "خارقة" في عالم التغذية
|
|
|
|
|
|
|
أمين عام أوبك: النفط الخام والغاز الطبيعي "هبة من الله"
|
|
|
|
|
|
|
قسم شؤون المعارف ينظم دورة عن آليات عمل الفهارس الفنية للموسوعات والكتب لملاكاته
|
|
|