
آخر المواضيع المضافة


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 6-7-2017
Date: 9-10-2020
Date: 28-5-2017
|
With an alkyl amine the lone pair electron is localized on the nitrogen. However, the lone pair electron on an amide are delocalized between the nitrogen and the oxygen through resonance. This makes amides much less basic compared to alkylamines.
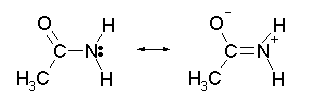
In fact,when and amide is reacted with an acid, the protonation occurs at the carbonyl oxygen and not the nitrogen. This is because the cation resulting from oxygen protonation is resonance stabilized. The cation resulting for the protonation of nitrogen is not resonance stabilized.



|
|
|
|
التوتر والسرطان.. علماء يحذرون من "صلة خطيرة"
|
|
|
|
|
|
|
مرآة السيارة: مدى دقة عكسها للصورة الصحيحة
|
|
|
|
|
|
|
نحو شراكة وطنية متكاملة.. الأمين العام للعتبة الحسينية يبحث مع وكيل وزارة الخارجية آفاق التعاون المؤسسي
|
|
|