


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
أقرأ أيضاً
التاريخ: 20-12-2021
التاريخ: 4-2-2019
التاريخ: 12-7-2018
التاريخ: 28-11-2019
|
When an SN1 reaction is carried out starting with a single pure enantiomer, such as D-2-chlorobutane, the product usually is a mixture of the enantiomeric substitution products with a slight predominance of that isomer which corresponds to inversion. Theoretically, a carbocation is expected to be most stable in the planar configuration and hence should lead to exactly equal amounts of the two enantiomers, regardless of the chiral configuration of the starting material (Figure 8-3). However, the extent of configuration change that actually results in an SN1 reaction depends upon the degree of "shielding" of the front side of the reacting carbon by the leaving group and its associated solvent molecules. If the leaving group does not get away from the carbocation before the product-determining step takes place, there will be some preference for nucleophilic attack at the back side of the carbon, which results in a predominance of the product of inverted configuration.
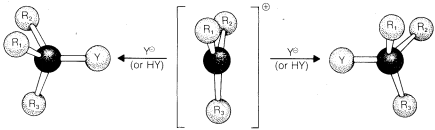
Other things being equal, the amount of inversion decreases as the stability of the carbocation intermediate increases, because the more stable the ion the longer is its lifetime, and the more chance it has of getting away from the leaving anion and becoming a relatively "free" ion. The solvent usually has a large influence on the stereochemical results of SN1 reactions because the stability and lifetime of the carbocations depend upon the nature of the solvent .
An orbital picture of SN1 ionization leading to a racemic product may be drawn as follows:
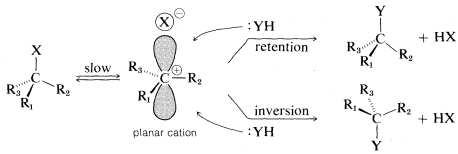
It should be clear that complete racemization is unlikely to be observed if X⊖ stays in close proximity to the side of the positive carbon that it originally departed from. We can say that X⊖ "shields" the front side, thereby favoring a predominance of inversion. If X⊖ gets far away before :YH comes in, then there should be no favoritism for one or the other of the possible substitutions.
If X⊖ and the carbocation, R⊕, stay in close proximity, as is likely to be the case in a solvent that does not promote ionic dissociation, then a more or less "tight" ion pair is formed, R⊕⋅⋅⋅X⊖. Such ion pairs often play an important role in ionic reactions in solvents of low dielectric constant .



|
|
|
|
للعاملين في الليل.. حيلة صحية تجنبكم خطر هذا النوع من العمل
|
|
|
|
|
|
|
"ناسا" تحتفي برائد الفضاء السوفياتي يوري غاغارين
|
|
|
|
|
|
|
المجمع العلمي يقيم ورشة تطويرية ودورة قرآنية في النجف والديوانية
|
|
|