


 النبات
النبات
 الحيوان
الحيوان
 الأحياء المجهرية
الأحياء المجهرية
 علم الأمراض
علم الأمراض
 التقانة الإحيائية
التقانة الإحيائية
 التقنية الحيوية المكروبية
التقنية الحيوية المكروبية
 التقنية الحياتية النانوية
التقنية الحياتية النانوية
 علم الأجنة
علم الأجنة
 الأحياء الجزيئي
الأحياء الجزيئي
 علم وظائف الأعضاء
علم وظائف الأعضاء
 الغدد
الغدد
 المضادات الحيوية
المضادات الحيوية|
Read More
Date: 29-2-2016
Date: 24-12-2020
Date: 29-2-2016
|
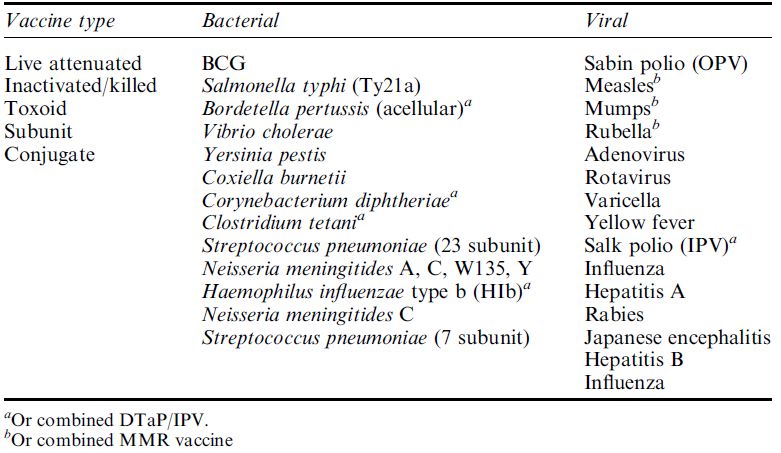
Types of Vaccines in Current Use
Current vaccines approved for use in humans fall into three main types: live, attenuated or inactivated whole organism vaccines; subunit vaccines, either isolated macromolecules or recombinant proteins; and toxoid vaccines. The major vaccines in current use are listed in Table.
1. Live, Attenuated Vaccines
These vaccines use attenuated strains of the pathogen. The attenuated strain is able to replicate inside the host but has lost one or more features which contribute to its pathogenicity. Attenuation can be achieved by growing the pathogen under abnormal culture conditions. The mutants generated through this process are then tested for loss of virulence while retaining immunogenicity. Attenuation by this approach can be a long and time-consuming process. The current vaccine for tuberculosis, bacillus Calmette–Gue´ rin (BCG), an attenuated strain of Mycobacterium bovis, was isolated by Calmette and Gue´ rin in 1921 after 13 years of growing M. bovis in medium containing increasing concentrations of bile. M. bovis confers immunity to M. tuberculosis, the primary cause of tuberculosis in humans due to similarities between the two organisms.
Although primarily associated with tuberculosis in cattle, M. bovis was also a significant cause of tuberculosis in humans prior to the introduction of pasteurised milk. The BCG vaccine is the most commonly administered vaccine, with over 5 billion doses having been given since its introduction in the 1940s. However, the efficacy of BCG is variable due in part to the fact that BCG, per se, has never been cloned and consequently there are variations between BCG preparations.
Attenuated viral vaccines are more common, but the basic strategy has been the same. Typically, attenuated strains of the virus are isolated by passage through cells which are not the normal host cell. The Sabin polio vaccine contains three different attenuated strains of poliovirus developed by growth in monkey kidney epithelial cells. Attenuated strains of the viruses responsible for measles, mumps and rubella were produced by similar approaches. These attenuated strains are used in the combined MMR vaccine. The attenuated measles virus may also used on its own; consequently, the WHO data refer to measles-containing vaccine (MCV).
The molecular attenuation or deletion of virulence genes should permit more rapid production of attenuated strains for use of vaccines. One current example is a typhoid vaccine, which uses the attenuated Salmonella typhi strain Ty21a. Recently, the production of a biologically contained Ebola virus has been reported. The gene encoding an essential viral transcription factor (VP30) required for viral replication was replaced by a reporter gene. Vero cells expressing the critical VP30 protein permit completion of the viral life cycle but the virus is contained within the cell type. These VP30-negative viruses are genetically stable and morphologically indistinguishable from the wild-type virus. The production of this biologically contained form of Ebola virus will permit studies of the virus outside of biosafety level-4 containment and should accelerate vaccine development for this highly lethal and feared virus.
2. Inactivated Vaccines
Several inactivated vaccines are in current use in humans (Table ). The approach is chemical inactivation of the pathogen using formaldehyde or b-propiolactone. This approach usually ensures that the immunogenic features of the pathogen are retained. Production of inactivated vaccines requires large quantities of pathogenic material, with associated risks for the workers involved. Furthermore, it is essential tha the inactivation process is complete, otherwise the pathogen will be introduced into the population. This occurred with early batches of the Salk polio vaccine, resulting in paralytic polio in a number of recipients.
The advantage over live, attenuated vaccines is that there is no risk of reversion to virulence. It is for this reason that the Salk vaccine is preferred over the Sabin polio vaccine in Scandinavian countries.
Inactivated vaccines generally produce significant antibody response but weaker cellular immunity, in particular cytotoxic T cell responses, than live vaccines. This is due mainly to the absence of microbial protein synthesis inside host cells. In turn, this leads to reduced or absent
cytosolic processing required for MHC class I presentation of peptides and poor activation of cytotoxic CD8+ T cells. Furthermore, the inability to replicate inside the host reduces immunostimulation and a need for more booster immunisations.
Table . The main types of bacterial and viral vaccines for use in humans



|
|
|
|
دراسة يابانية لتقليل مخاطر أمراض المواليد منخفضي الوزن
|
|
|
|
|
|
|
اكتشاف أكبر مرجان في العالم قبالة سواحل جزر سليمان
|
|
|
|
|
|
|
المجمع العلمي ينظّم ندوة حوارية حول مفهوم العولمة الرقمية في بابل
|
|
|