
 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 19-10-2018
Date: 26-12-2018
Date: 16-1-2018
|
The two most common compounds of nitrogen are Potassium Nitrate (KNO3) and Sodium Nitrate (NaNO3). These two compounds are formed by decomposing organic matter that has potassium or sodium present and are often found in fertilizers and byproducts of industrial waste. Most nitrogen compounds have a positive Gibbs free energy (i.e., reactions are not spontaneous).
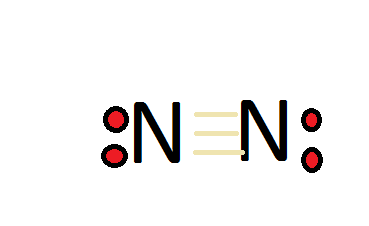
Figure 1.1
: Lewis Dot structure of molecular nitrogen
The dinitrogen molecule (N2) is an "unusually stable" compound, particularly because nitrogen forms a triple bond with itself. This triple bond is difficult hard to break. For dinitrogen to follow the octet rule, it must have a triple bond. Nitrogen has a total of 5 valence electrons, so doubling that, we would have a total of 10 valence electrons with two nitrogen atoms. The octet requires an atom to have 8 total electrons in order to have a full valence shell, therefore it needs to have a triple bond. The compound is also very inert, since it has a triple bond. Triple bonds are very hard to break, so they keep their full valence shell instead of reacting with other compounds or atoms. Think of it this way, each triple bond is like a rubber band, with three rubber bands, the nitrogen atoms are very attracted to each other.



|
|
|
|
تفوقت في الاختبار على الجميع.. فاكهة "خارقة" في عالم التغذية
|
|
|
|
|
|
|
أمين عام أوبك: النفط الخام والغاز الطبيعي "هبة من الله"
|
|
|
|
|
|
|
قسم شؤون المعارف ينظم دورة عن آليات عمل الفهارس الفنية للموسوعات والكتب لملاكاته
|
|
|