
Structure of the Alkyl Group, R, in SN1 Reactions
 المؤلف:
John D. Roberts and Marjorie C. Caserio
المؤلف:
John D. Roberts and Marjorie C. Caserio
 المصدر:
Basic Principles of Organic Chemistry : LibreTexts project
المصدر:
Basic Principles of Organic Chemistry : LibreTexts project
 الجزء والصفحة:
........
الجزء والصفحة:
........
 5-1-2022
5-1-2022
 2748
2748
Structure of the Alkyl Group, R, in SN1 Reactions
The rates of SN1 reactions of simple alkyl derivatives follow the order tertiary R ≫ secondary R > primary R, which is exactly opposite that of SN2 reactions. This is evident from the data in Table 8-2, which lists the relative rates of hydrolysis of some alkyl bromides; only the secondary and tertiary bromides react at measurable rates, and the tertiary bromide reacts some 105 times faster than the secondary bromide.
Why do tertiary alkyl compounds ionize so much more rapidly than either secondary or primary compounds? The reason is that tertiary alkyl cations are more stable than either secondary or primary cations and therefore are formed more easily. You will appreciate this better by looking at the energy diagram of Figure 8-4, which shows the profile of energy changes for hydrolysis of an alkyl compound, RX, by the SN1 mechanism. The rate of
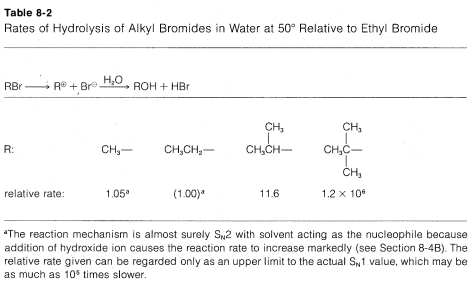

Figure 8-4: Profile of energy changes for hydrolysis of RX by the SN1 mechanism in accord with the steps

The last step is not part of the SN1 process itself but is included for completeness. The water molecule shown in parentheses (+H2O) is necessary to balance the equations, but it should not be considered to be different from the other water molecules in the solvent until it is specifically involved in the second transition state.
reaction is determined by the ionization step, or by the energy of the transition state relative to that of the reactants. Actually, the energy of the transition state is only slightly higher than the energy of the ionic intermediates R⊕X⊖. Thus to a first approximation, we can say that the rate of ionization of RX will depend on the energies of the ions formed. Now if we compare the rates for a series of compounds, RX, all having the same leaving group, X, but differing only in the structure of RR, their relative rates of ionization will correspond to the relative stabilities of R⊕. The lower energy of R⊕, the faster will be the rate of ionization. Therefore the experimental results suggest that the sequence of carbocation stabilities is tertiary R⊕ ≫ secondary R⊕ ≫ primary R⊕.
Just why this sequence is observed is a more difficult question to answer. Notice in the following stability sequence that alkyl cations are more stable the more alkyl groups there are on the positive carbon:
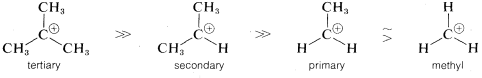
The simplest explanation for why this is so is that alkyl groups are more polarizable than hydrogens. In this case, more polarizable means the electrons of the alkyl groups tend to move more readily toward the positive carbon than do those of the hydrogens. Such movements of electrons transfer part of the charge on the cationic carbon to the alkyl groups, thereby spreading the charge over a greater volume. This constitutes electron delocalization, which results in enhanced stability.
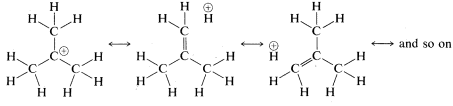
An alternative way of explaining how the cationic charge is spread over the alkyl groups of a tertiary cation, such as the tert-butyl cation, is to write the cation as a hybrid of the following structures:
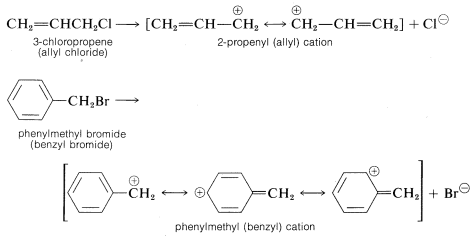
Other organohalogen compounds besides secondary and tertiary alkyl compounds can react by SN1 mechanisms provided they have the ability to form reasonably stabilized carbon cations. Examples include 2-propenyl (allylic) and phenylmethyl (benzylic) compounds, which on ionization give cations that have delocalized electrons:
In general, the more stabilized the carbon cation from an alkyl halide, the more reactive the compound will be in SN1-type reactions. This is especially apparent in the reactivities of compounds with phenyl groups on the reacting carbon. As the number of phenyl groups increases from zero to three, the SN1 reactivity of the chlorides increases by more than 107 because of increasing stabilization of the carbon cation by the phenyl groups:
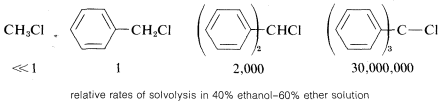
In contrast, compounds such as chlorobenzene and chloroethene, in which the halogen is attached directly to a multiply bonded carbon atom, do not exhibit SN1-type reactions. Evidently then, unsaturated carbon cations such as phenyl or ethenyl are appreciably less stable (more difficult to form) than tert-alkyl cations:

Steric hindrance is relatively unimportant in SN1 reactions because the rate is independent of the nucleophile. In fact, steric acceleration is possible in the solvolysis of highly branched alkyl halides through relief of steric compression between the alkyl groups in the halide by formation of a planar cation:
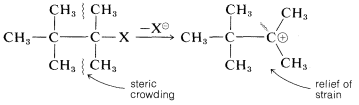
Along with the effect R has on the rate at which an alkyl compound RX reacts by an SN1 mechanism, the group R also affects the nature of the products obtained. The intermediate alkyl cations R⊕ may react in various ways to give products of substitution, elimination, and rearrangement.
 الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


