
The Question of the Equilibrium Constant
 المؤلف:
John D. Roberts and Marjorie C. Caserio
المؤلف:
John D. Roberts and Marjorie C. Caserio
 المصدر:
Basic Principles of Organic Chemistry : LibreTexts project
المصدر:
Basic Principles of Organic Chemistry : LibreTexts project
 الجزء والصفحة:
........
الجزء والصفحة:
........
 23-12-2021
23-12-2021
 2034
2034
The Question of the Equilibrium Constant
Presumably, methane could react with chlorine to give chloromethane and hydrogen chloride, or chloromethane could react with hydrogen chloride to give methane and chlorine. If conditions were found for which both reactions proceeded at a finite rate, equilibrium finally would be established when the rates of the reactions in each direction became equal
CH4+Cl2⇌CH3Cl+HCl (4.5.2)
At equilibrium, the relationship among the amounts of reactants and products is given by the equilibrium constant expression
Keq=[CH3Cl][HCl]/[CH4][Cl2] (4.5.3)
in which KeqKeq is the equilibrium constant.
The quantities within the brackets of Equation 4.5.3 denote either concentrations for liquid reactants or partial pressures for gaseous substances. If the equilibrium constant Keq is greater than 1, then on mixing equal volumes of each of the participant substances (all are gases above −24o), reaction to the right will be initially faster than reaction to the left, until equilibrium is established; at this point there will be more chloromethane and hydrogen chloride present than methane and chlorine. However, if the equilibrium constant were less than 1, the reaction initially would proceed faster to the left and, at equilibrium, there would be more methane and chlorine present than chloromethane and hydrogen chloride. For methane chlorination, we know from experiment that the reaction goes to the right and that KeqKeq is much greater than unity. Naturally, it would be helpful in planning other organic preparations to be able to estimate KeqKeq in advance.
It is a common experience to associate chemical reactions with equilibrium constants greater than one with the evolution of heat, in other words, with negative ΔH0 values. There are, in fact, many striking examples. Formation of chloromethane and hydrogen chloride from methane and chlorine has a Keq of 1018 and ΔH0 of −24 kcal per mole of CH3Cl formed at 25o. Combustion of hydrogen with oxygen to give water has a KeqKeq of 1040 and ΔH0=−57 kcal per mole of water formed at 25o. However, this correlation between KeqKeq and ΔH0 is neither universal nor rigorous. Reactions are known that absorb heat (are endothermic) and yet have Keq>1. Other reactions have large ΔH0 values and equilibrium constants much less than 1.
The problem is that the energy change that correlates with Keq is not ΔH0 but ΔG0 (the so-called change of "standard Gibbs energy"), and if we know ΔG0, we can calculate KeqKeq by the equation

in which R is the gas constant and T is the absolute temperature in degrees Kelvin. For our calculations, we shall use R as 1.987caldeg−1mol−1 and you should not forget to convert ΔG0 to calcal.
Tables of ΔG0 values for formation of particular compounds (at various temperatures and states) from the elements are available in handbooks and the literature. With these, we can calculate equilibrium constants quite accurately. For example, handbooks give the following data, which are useful for methane chlorination:
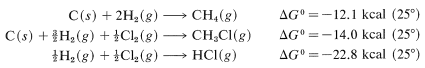
Combining these with proper regard for sign gives
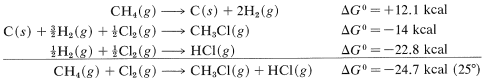
and logKeq=−(−24.7×1000)/(2.303×1.987×298.2), so Keq=1.3×1018. Unfortunately, insufficient ΔG0 values for formation reactions are available to make this a widely applicable method for calculating KeqKeq values.
The situation is not wholly hopeless, because there is a relationship between ΔG0 and ΔH0 that also involves T and another quantity, ΔS0, the standard entropy change of the process
This equation shows that ΔG0 and ΔH0 are equal when ΔS0 is zero. Therefore the sign and magnitude of TΔS0 determine how well KeqKeq correlates with ΔH0. Now, we have to give attention to whether we can estimate TΔS0 values well enough to decide whether the ΔH0 of a given reaction (calculated from bond energies or other information) will give a good or poor measure of ΔG0. :

 الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


