
Entropy and Molecular Disorder
 المؤلف:
John D. Roberts and Marjorie C. Caserio
المؤلف:
John D. Roberts and Marjorie C. Caserio
 المصدر:
Basic Principles of Organic Chemistry : LibreTexts project
المصدر:
Basic Principles of Organic Chemistry : LibreTexts project
 الجزء والصفحة:
........
الجزء والصفحة:
........
 23-12-2021
23-12-2021
 2221
2221
Entropy and Molecular Disorder
To decide whether we need to worry about ΔS0 with regard to any particular reaction, we have to have some idea what physical meaning entropy has. To be very detailed about this subject is beyond the scope of this book, but you should try to understand the physical basis of entropy, because if you do, then you will be able to predict at least qualitatively whether ΔH0 will be about the same or very different from ΔG0. Essentially, the entropy of a chemical system is a measure of its molecular disorder or randomness. Other things being the same, the more random the system is, the more favorable the system is.
Different kinds of molecules have different degrees of translational, vibrational, and rotational freedom and, hence, different average degrees of molecular disorder or randomness. Now, if for a chemical reaction the degree of molecular disorder is different for the products than for the reactants, there will be a change in entropy and ΔS0≠0.
A spectacular example of the effect of molecular disorder in contributing to the difference between ΔH0 and ΔG0 is afforded by the formation of liquid nonane, C9H20, from solid carbon and hydrogen gas at 25o:

with ΔHo=−54.7kcal and ΔSo=5.0kcal.
Equations 4.5.4 and 4.5.5 can be rearranged to calculate ΔS0 and Keq from ΔH0 and ΔG0
and

These ΔH0, ΔS0, and Keq values can be compared to those for H2+12O2⟶H2O, for which ΔH0 is −57kcal, ΔS0 is 8.6e.u., and Keq is 1040. Obviously, there is something unfavorable about the entropy change from carbon and hydrogen to nonane. The important thing is that there is a great difference in the constraints on the atoms on each side of the equation. In particular, hydrogen molecules in the gaseous state have great translational freedom and a high degree of disorder, the greater part of which is lost when the hydrogen atoms become attached to a chain of carbons. This makes for a large negative ΔS0, which corresponds to a decrease in KeqKeq. The differences in constraints of the carbons are less important. Solid carbon has an ordered, rigid structure with little freedom of motion of the individual carbon atoms. These carbons are less constrained in nonane, and this would tend to make ΔS0 more positive and ΔG0ΔG0 more negative, corresponding to an increase in Keq (Equations 4.5.4 and 4.5.5). However, this is a small effect on ΔS0 compared to the enormous difference in the degree of disorder of hydrogen between hydrogen gas and hydrogen bound to carbon in nonane.
Negative entropy effects usually are observed in ring-closure reactions such as the formation of cyclohexane from 1-hexene, which occur with substantial loss of rotational freedom (disorder) about the C−C bonds
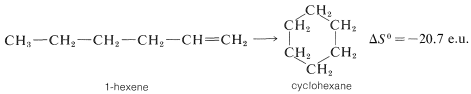
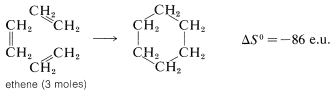
There is an even greater loss in entropy on forming cyclohexane from ethene because substantially more freedom is lost in orienting three ethene molecules to form a ring:
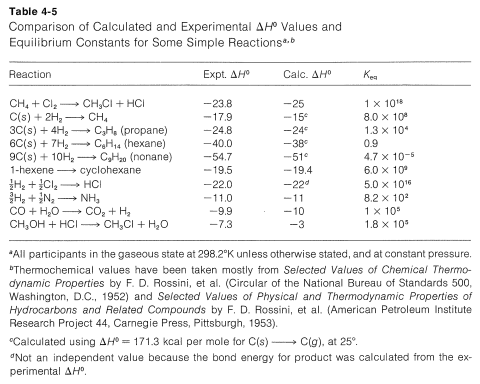
For simple reactions, with the same number of molecules on each side of the equation, with no ring formation or other unusual changes in the constraints between the products and reactants, ΔS0ΔS0 usually is relatively small. In general, for such processes, we know from experience that KeqKeq usually is greater than 1 if ΔH0 is more negative than −15kcal and usually is less than 1 for ΔH0 more positive than +15kcal. We can use this as a "rule of thumb" to predict whether KeqKeq should be greater or less than unity for vapor-phase reactions involving simple molecules. Some idea of the degree of success to be expected from this rule may be inferred from the examples in Table 4-5, which also contains a further comparison of some experimental ΔH0 values with those calculated from bond energies.
Suppose ΔG0 is positive, what hope do we have of obtaining a useful conversion to a desired product? There is no simple straightforward and general answer to this question. When the reaction is reversible the classic procedure of removing one or more of the products to prevent equilibrium from being established has many applications in organic chemistry, as will be seen later. When this approach is inapplicable, a change in reagents is necessary. Thus, iodine does not give a useful conversion with 2,2-dimethylpropane, 1, to give 1-iodo-2,2-dimethylpropane, 2, because the position of equilibrium is too far to the left (Keq≅10−5):
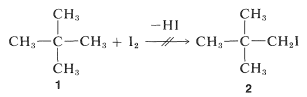
Alternative routes with favorable ΔG0 values are required. Development of ways to make indirectly, by efficient processes, what cannot be made directly is one of the most interesting and challenging activities of organic chemists.
 الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


