
Meerwein-Pondorf-Verley Reduction
 المؤلف:
William Reusch
المؤلف:
William Reusch
 المصدر:
Virtual Textbook of Organic Chemistry
المصدر:
Virtual Textbook of Organic Chemistry
 الجزء والصفحة:
............
الجزء والصفحة:
............
 5-8-2018
5-8-2018
 1906
1906
Meerwein-Pondorf-Verley Reduction
Reduction of aldehydes and ketones to alcohols is most commonly carried out by metal hydride reagents, dissolving metal reagents, and sometimes by catalytic hydrogenation. A table listing these reagents and the conditions under which they are best used as show in below.
Reducing Reagents
| Reagent |
Preferred Solvents |
Functions Reduced |
Reaction Work-up |
Sodium Borohydride
NaBH4 |
ethanol; aqueous ethanol
15% NaOH; diglyme
avoid strong acids |
aldehydes to 1º-alcohols
ketones to 2º-alcohols
1,2-reduction of enones is favored by CeCl3
inert to most other functions |
1) simple neutralization
2) extraction of product |
Lithium Aluminum Hydride (LAH)
LiAlH4 |
ether; THF
avoid alcohols and amines
avoid halogenated compounds
avoid strong acids |
aldehydes to 1º-alcohols
ketones to 2º-alcohols
carboxylic acids to 1º-alcohols
esters to alcohols
epoxides to alcohols
nitriles & amides to amines
halides & tosylates to alkanes
most functions react |
1) careful addition of water
2) dissolve aluminum salts
3) extraction of product |
Lithium tri t-Butoxyaluminohydride
LiAlH(Ot-C4H9)3 |
ether; THF
avoid alcohols and amines
avoid halogenated compounds
avoid strong acids |
fast:
acid chlorides to aldehydes
(at -78 ºC)
3º-amides to aldehydes (at -78 ºC)
nitriles to aldehydes (at -78 ºC)
slower:
aldehydes to 1º-alcohols
ketones to 2º-alcohols |
1) careful addition of water
2) dissolve aluminum salts
3) extraction of product |
Diisobutylaluminum Hydride
AlH[CH2CH(CH3)2]2 |
THF; toluene
avoid alcohols and amines
avoid halogenated compounds
avoid strong acids |
fast:
acid chlorides to aldehydes
(at -78 ºC)
3º-amides to aldehydes (at -78 ºC)
nitriles to aldehydes (at -78 ºC)
slower:
aldehydes to 1º-alcohols
ketones to 2º-alcohols |
1) careful addition of water
2) dissolve aluminum salts
3) extraction of product |
Diborane
B2H6 = 2 BH3 |
ether; THF
sulfide complex in CH2Cl2
complexes with amines
avoid alkenes & alkynes |
carboxylic acids to 1º-alcohols
aldehydes to 1º-alcohols
ketones to 2º-alcohols
nitriles to amines
esters & epoxides slowly reduced |
1) dilute acid or H2O2
2) extraction of product |
|
Hydrogen & Catalyst
H2 & Pt, or Pd, or Ru, or Ni
Modified (poisoned) Catalyst
|
alcohols, ethers, hydrocarbons
or carboxylic acids |
alkenes & alkynes to alkanes (fast)
nitro groups to amines (fast)
imines to amines (fast)
aldehydes & ketones to alcohols (slow)
nitriles to amines (slow)
may remove benzylic groups
alkynes to alkenes
acyl chlorides to aldehydes
|
filter to remove catalyst |
|
Reactive Metals
Na, or Li, or K
Mg or Al or Zn or Fe
|
liq. ammonia & ether co-solvents
or alcohols or amines
water, alcohols, acetic acid
or aqueous mineral acid
|
ketones to 2º-alcohols
alkynes to alkenes
conjugated π-systems
(e.g. aromatic rings, dienes & enones)
cleaves C-X and benzylic groups
cleaves activated substituents
nitro groups to amines
C=O (aldehyde/ketone) to CH2
|
1) quench with NH4Cl
2) extract product
extract product from salts
|
Prior to the development of these new and powerful reduction methods, the conversion of carbonyl compounds to alcohols was often effected by hydrogen transfer from an alkoxide salt. This procedure, known as the Meerwein-Pondorf-Verley reaction, is illustrated by the following equation and mechanism ( the hydride-like hydrogen is colored red). Aluminum isopropoxide has been the most common hydrogen source in most cases, but lanthanide salts, such as ROSmI2 have been used with good results. This reduction is specific for aldehyde and ketone carbonyl functions, so other easily reduced functions such as nitro groups and halogen are unaffected.

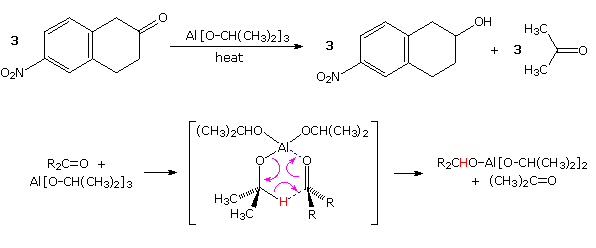
Not only are two hydrogens delivered independently from the least hindered (convex) side of the cis-decalin substrate in example 1, but the easily reduced double bond of the enedione remains unchanged. The initially formed cis-diol undergoes lactonization with the neighboring methyl ester. It should be noted that a similar reductive hydride transfer takes place when large alkyl Grignard reagents react with hindered ketones, as shown in equation 2.
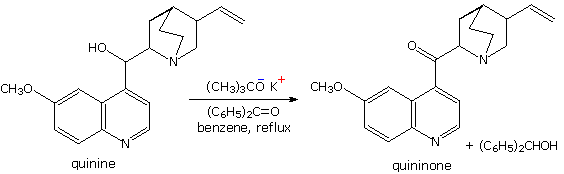
The MVP reduction is also an oxidation, as evidenced by the conversion of isopropoxide to acetone. Consequently, the reaction can be converted into an oxidation of alcohols to ketones or aldehydes. This procedure is called the Oppenauer oxidation. The reaction displayed below is an example of the Oppenauer oxidation in which benzophenone is the oxidant. Two significant features may be noted. First, the oxidation is specific for alcohols, and does not oxidize other sensitive functions such as amines and sulfides. Second, although aluminum or other coordinating metals are often used as cationic partners, alkali metals alone will suffice.
 الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


