


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 7-7-2020
Date: 13-11-2020
Date: 23-2-2017
|
Oxides and oxoacids of carbon
Unlike the later elements in group 14, carbon forms stable, volatile monomeric oxides: CO and CO2. A comment on the difference between CO2 and SiO2 can be made in the light of the thermochemical data in Table 1.1: the C=O bond enthalpy term is more than twice that for the C_O bond, while the Si=O bond enthalpy term is less than twice that of the Si_O bond.
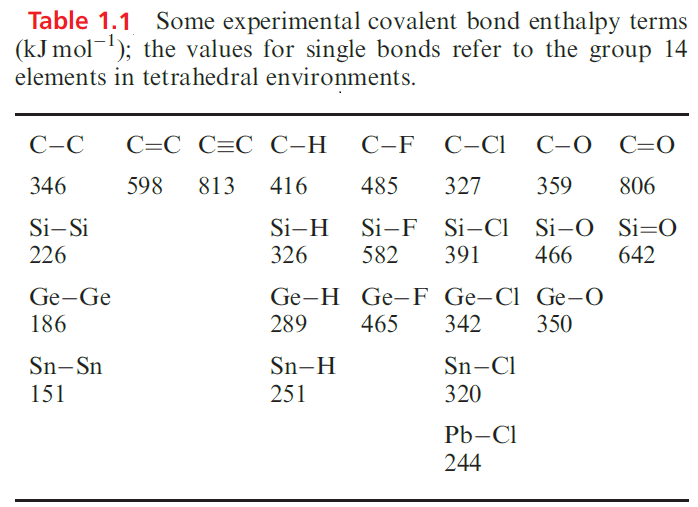
In rationalizing these differences, there is some justification for saying that the C=O bond is strengthened relative to Si=O by (p–p)π contributions, and, in the past, it has been argued that the Si_O bond is strengthened relative to the C_O bond by ( p–d)π-bonding.
Irrespective of the interpretation of the enthalpy terms however, the data indicate that (ignoring enthalpy and entropy changes associated with vaporization) SiO2 is stable with respect to conversion into molecular O=Si=O, while CO2 is stable with respect to the formation of a macromolecular species containing 4-coordinate C and C_O single bonds. However, an extended solid phase of CO2 has recently been prepared by laser-heating a molecular phase at 1800K and under 40 GPa pressure; the vibrational spectrum of the new phase indicates that it is structurally similar to quartz . Carbon monoxide is a colourless gas, formed when C burns in a restricted supply of O2. Small-scale preparations involve the dehydration of methanoic acid (as in below equation). CO is manufactured by reduction of CO2 using coke heated above 1070K or by the water–gas shift reaction.
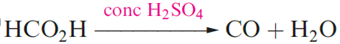
Carbon monoxide is almost insoluble in water under normal conditions and does not react with aqueous NaOH, but at high pressures and temperatures, HCO2H and Na[HCO2] are formed respectively. Carbon monoxide combines with F2, Cl2 and Br2 sulfur and selenium. The high toxicity of CO arises from the formation of a stable complex with haemoglobin (see Section 28.3) with the consequent inhibition of O2 transport in the body. The oxidation of CO to CO2 is the basis of quantitative analysis for CO with the I2 formed being removed and titrated against thiosulfate. CO is similarly oxidized by a mixture of MnO2, CuO and Ag2O at ambient temperatures and this reaction is used in respirators.
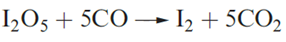
The thermodynamics of the oxidation of carbon is of immense importance in metallurgy. Selected physical properties of CO and CO2 are given in B Table 1.2; bonding models . The bond in CO is the strongest known in a stable molecule and confirms the efficiency of ( p–p)π-bonding between C and O. However, considerations of the bonding provide no simple explanation as to why the dipole moment of CO is so low. In an excess of O2, C burns to give CO2. Solid CO2 is called dry ice and readily sublimes (Table 1.2) but may be kept in insulated containers for laboratory use in, e.g. lowtemperature baths (Table 1.3).
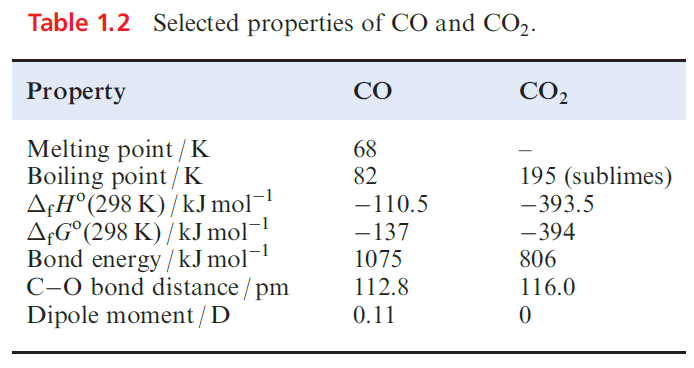

Supercritical CO2 has become a much studied and versatile solvent. Small-scale laboratory syntheses of gaseous CO2 usually involve reactions.

Carbon dioxide is the world’s major environmental source of acid and its low solubility in water is of immense biochemical and geochemical significance. In an aqueous solution of carbon dioxide, most of the solute is present as molecular CO2 rather than as H2CO3, as can be seen from the value of K ≈ 1.7 × 10-3 for the equilibrium:
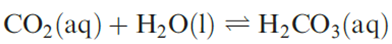
Aqueous solutions of CO2 are only weakly acidic, but it does not follow that H2CO3 (carbonic acid) is a very weak acid. The value of pKa(1) for H2CO3 is usually quoted.
This evaluation, however, assumes that all the acid is present in solution as H2CO3 or [HCO3]- when, in fact, a large proportion is present as dissolved CO2. By taking this into account, one arrives at a ‘true’ pKa(1) for H2CO3 of ≈3.6. Moreover, something that is of great biological and industrial importance is the fact that combination of CO2 with water is a relatively slow process. This can be shown by titrating a saturated solution of CO2 against aqueous NaOH using phenolphthalein as indicator. Neutralization of CO2 occurs by two routes. For pH<8, the main pathway is by direct hydration which shows pseudo-first order kinetics. At pH>10, the main pathway is by attack of hydroxide ion.
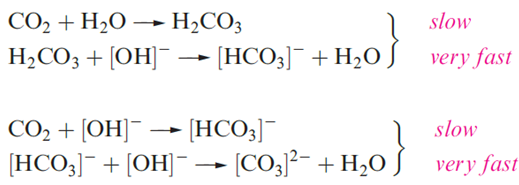
Until 1993, there was no evidence that free carbonic acid had been isolated, although an unstable ether adduct is formed when dry HCl reacts with NaHCO3 suspended in Me2O at 243 K, and there is mass spectrometric evidence for H2CO3 being a product of the thermal decomposition of [NH4][HCO3]. However, IR spectroscopic data now indicate that H2CO3 can be isolated using a cryogenic method in which glassy MeOH solution layers of KHCO3 (or Cs2CO3) and HCl are quenched on top of each other at 78K and the reaction mixture warmed to 300 K. In the absence of water, H2CO3 can be sublimed unchanged. It remains a fact that, under ambient conditions, H2CO3 is not a readily studied species.
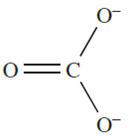
The carbonate ion is planar and possesses D3h symmetry with all C_O bonds of length 129 pm. A delocalized bonding picture involving ( p–p)π-interactions is appropriate, and VB theory describes the ion in terms of three resonance. The C_O bond distance in [CO3]2- is longer than in CO2 (Table 1.1) and is consistent with a formal bond order of 1.13. Most metal carbonates, other than those of the group 1 metals are sparingly soluble in water. A general method of preparing peroxo salts can be used to convert K2CO3 to K2C2O6; the electrolysis of aqueous K2CO3 at 253K using a high current density produces a salt believed to contain the peroxocarbonate ion.
An alternative route involves the reaction of CO2 with KOH in 86% aqueous H2O2 at 263 K. The colour of the product is variable and probably depends upon the presence of impurities such as KO3. The electrolytic method gives a blue material whereas the product from the second route is orange. Peroxocarbonates are also believed to be intermediates in the reactions of CO2 with superoxides.

A third oxide of carbon is the suboxide C3O2 which is made by dehydrating malonic acid, CH2(CO2H)2, using P2O5 at 430 K. At room temperature, C3O2 is a gas (bp 279 K), but it polymerizes above 288K to form a red-brown paramagnetic material. The structure of C3O2 is usually described as ‘quasi-linear’ because IR spectroscopic and electron diffraction data for the gaseous molecule show that the energy barrier to bending at the central C atom is only 0.37 kJ mol_1, i.e. very close to the vibrational ground state. The melting point of C3O2 is 160 K. An X-ray diffraction study of crystals grown just below this temperature confirms that the molecules are essentially linear in the solid state. However, the data are best interpreted in terms of disordered bent molecules with a C_C_C bond angle close to 1708, consistent with a ‘quasi-linear’ description.
The species
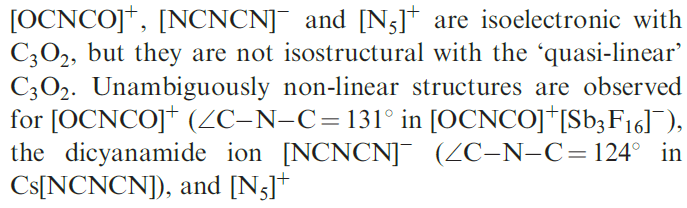



|
|
|
|
دخلت غرفة فنسيت ماذا تريد من داخلها.. خبير يفسر الحالة
|
|
|
|
|
|
|
ثورة طبية.. ابتكار أصغر جهاز لتنظيم ضربات القلب في العالم
|
|
|
|
|
|
|
العتبة العباسية المقدسة تدعو جامعة ديالى للمشاركة في حفل التخرج المركزي الخامس
|
|
|