


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 22-12-2015
Date: 14-9-2017
Date: 31-8-2017
|
Aniline (C6H5NH2)
Aniline (aminobenzene) is an oily liquid that turns brown when exposed to air and light. The compound is an important dye precursor. The main process for producing aniline is the hydrogenation of nitrobenzene:
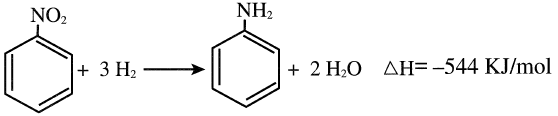
The hydrogenation reaction occurs at approximately 270°C and slightly above atmospheric over a Cu/Silica catalyst. About a 95% yield is obtained.
An alternative way to produce aniline is through ammonolysis of either chlorobenzene or phenol. The reaction of chlorobenzene with aqueous ammonia occurs over a copper salt catalyst at approximately 210°C and 65 atmospheres. The yield of aniline from this route is also about 96%:
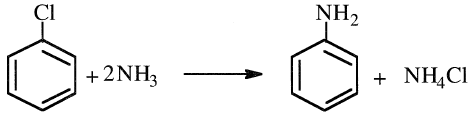
Ammonolysis of phenol occurs in the vapor phase. In the Scientific Design Co. process (Figure 1.1), a mixed feed of ammonia and phenol is heated and passed over a heterogeneous catalyst in a fixed-bed system. The reactor effluent is cooled, the condensed material distilled, and the unreacted ammonia recycled. Aniline produced this way should be very pure:

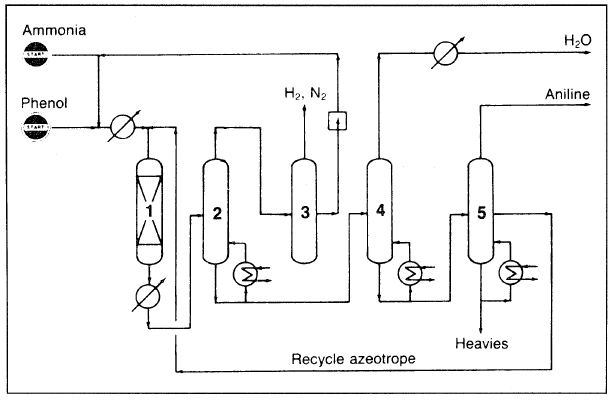
Figure 1.1. The Scientific Co. process for producing aniline from phenol: (1) fixed-bed reactor, (2) liquid-gas separator, (3) ammonia compression and recycling, (4) drier, (5) fractionator.



|
|
|
|
4 أسباب تجعلك تضيف الزنجبيل إلى طعامك.. تعرف عليها
|
|
|
|
|
|
|
أكبر محطة للطاقة الكهرومائية في بريطانيا تستعد للانطلاق
|
|
|
|
|
|
|
العتبة العباسية المقدسة تبحث مع العتبة الحسينية المقدسة التنسيق المشترك لإقامة حفل تخرج طلبة الجامعات
|
|
|