


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 23-11-2015
Date: 27-7-2017
Date: 16-8-2017
|
HYDRATION OF ETHYLENE
(Ethanol Production)
Ethyl alcohol (CH3CH2OH) production is considered by many to be the world’s oldest profession. Fermenting carbohydrates is still the main route to ethyl alcohol in many countries with abundant sugar and grain sources.
Synthetic ethyl alcohol (known as ethanol to differentiate it from fermentation alcohol) was originally produced by the indirect hydration of ethylene in the presence of concentrated sulfuric acid. The formed monoand diethyl sulfates are hydrolyzed with water to ethanol and sulfuric acid, which is regenerated:
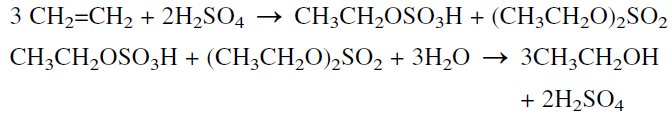
The direct hydration of ethylene with water is the process currently used:
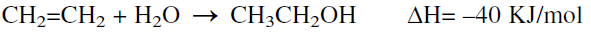
The hydration reaction is carried out in a reactor at approximately 300°C and 70 atmospheres. The reaction is favored at relatively lower temperatures and higher pressures. Phosphoric acid on diatomaceous earth is the catalyst. To avoid catalyst losses, a water/ethylene mole ratio less than one is used. Conversion of ethylene is limited to 4–5% under these conditions, and unreacted ethylene is recycled. A high selectivity to ethanol is obtained (95–97%).



|
|
|
|
كل ما تود معرفته عن أهم فيتامين لسلامة الدماغ والأعصاب
|
|
|
|
|
|
|
ماذا سيحصل للأرض إذا تغير شكل نواتها؟
|
|
|
|
|
|
|
جامعة الكفيل تناقش تحضيراتها لإطلاق مؤتمرها العلمي الدولي السادس
|
|
|