


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 11-3-2017
Date: 10-5-2020
Date: 3-1-2017
|
Geometrical Isomers
In some isomers, the connections of the atoms is the same, but the isomers differ because at least two atoms, bonded to the same or adjacent atoms but not to each other, are at different distances from one another. Such isomers are termed geometrical isomers. Some examples are shown in Figure 1.1.
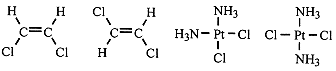
Figure 1.1. Examples of geometrical isomers.
ClHC=CHCl, 1,2-dichloroethylene, has two geometrical isomers which we term cis- (Cl atoms on the same side) and trans (Cl atoms on opposite sides). Similarly, Pt(NH3)2Cl2 has cis and trans isomers. The cis isomers have dipole moments (the polar C-Cl bonds have bond moments which add vectorially), but the trans isomers are nonpolar (the C-Cl bond moments cancel). Dichlorobenzene has three geometrical isomers, termed ortho (Cl atoms on adjacent C's), meta (Cl atoms on alternate C's) and para (Cl atoms on opposite C's). The para isomer is nonpolar and the ortho isomer has a large dipole moment; the meta isomer is intermediate in polarity.



|
|
|
|
5 علامات تحذيرية قد تدل على "مشكل خطير" في الكبد
|
|
|
|
|
|
|
لحماية التراث الوطني.. العتبة العباسية تعلن عن ترميم أكثر من 200 وثيقة خلال عام 2024
|
|
|