


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 13-4-2017
Date: 6-4-2017
Date: 11-9-2016
|
Buffers Are Mixtures of Weak Acids and Their Conjugate Bases
Buffers are aqueous systems that tend to resist changes in pH when small amounts of acid (H+) or base (OHˉ) are added. A buffer system consists of a weak acid (the proton donor) and its conjugate base (the proton acceptor). As an example, a mixture of equal concentrations of acetic acid and acetate ion, found at the midpoint of the titration curve in Figure 1.1, is a buffer system. The titration curve of acetic acid has a relatively flat zone extending about 1 pH unit on either side of its midpoint pH of 4.76.

Figure 1.1
In this zone, an amount of H+ or OHˉ added to the system has much less effect on pH than the same amount added outside the buffer range. This relatively flat zone is the buffering region of the acetic acid–acetate buffer pair. At the midpoint of the buffering region, where the concentration of the proton donor (acetic acid) exactly equals that of the proton acceptor (acetate), the buffering power of the system is maximal; that is, its pH changes least on addition of H+ or OHˉ. The pH at this point in the titration curve of acetic acid is equal to its pKa. The pH of the acetate buffer system does change slightly when a small amount of H+ or OHˉ is added, but this change is very small compared with the pH change that would result if the same amount of H+ or OHˉ were added to pure water or to a solution of the salt of a strong acid and strong base, such as NaCl, which has no buffering power. Buffering results from two reversible reaction equilibria occurring in a solution of nearly equal concentrations of a proton donor and its conjugate proton acceptor. Figure 1.2 explains how a buffer system works.
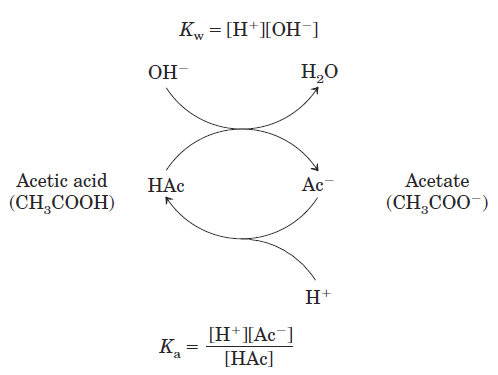
Figure 1.2
Whenever H+ or OHˉ is added to a buffer, the result is a small change in the ratio of the relative concentrations of the weak acid and its anion and thus a small change in pH. The decrease in concentration of one component of the system is balanced exactly by an increase in the other. The sum of the buffer components does not change, only their ratio. Each conjugate acid-base pair has a characteristic pH zone in which it is an effective buffer (Fig. 1.3).
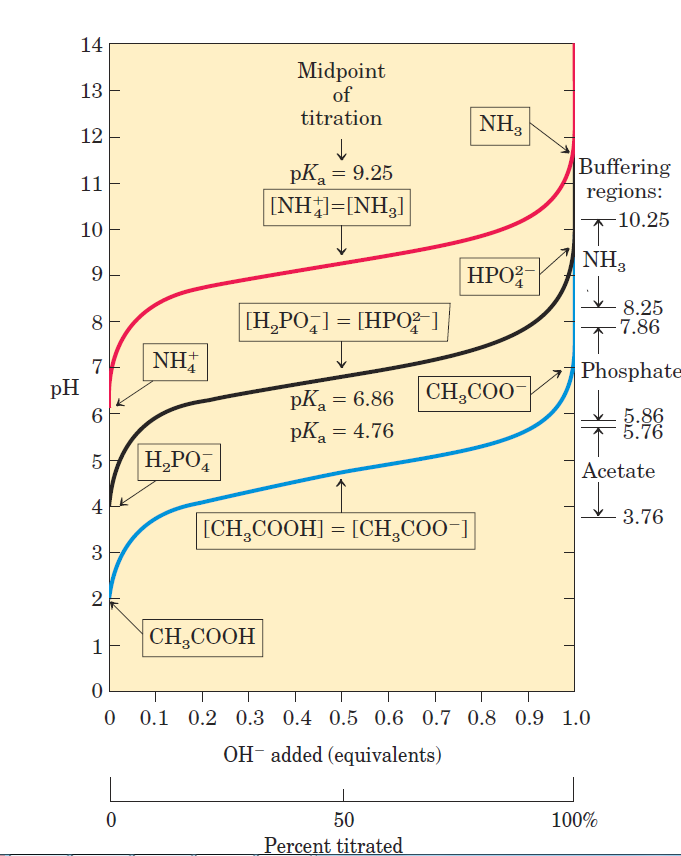
Figure 1.3
The H2PO4- /HPO42- pair has a pKa of 6.86 and thus can serve as an effective buffer system between approximately pH 5.9 and pH 7.9; the NH4- /NH3 pair, with a pKa of 9.25, can act as a buffer between approximately pH 8.3 and pH 10.3.



|
|
|
|
للعاملين في الليل.. حيلة صحية تجنبكم خطر هذا النوع من العمل
|
|
|
|
|
|
|
"ناسا" تحتفي برائد الفضاء السوفياتي يوري غاغارين
|
|
|
|
|
|
|
نحو شراكة وطنية متكاملة.. الأمين العام للعتبة الحسينية يبحث مع وكيل وزارة الخارجية آفاق التعاون المؤسسي
|
|
|