


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 13-4-2017
Date: 12-4-2017
Date: 26-7-2016
|
Amino Acids Can Be Classified by R Group
Knowledge of the chemical properties of the common amino acids is central to an understanding of biochemistry. The topic can be simplified by grouping the amino acids into five main classes based on the properties of their R groups (Table 1.1), in particular, their polarity, or tendency to interact with water at biological pH (near pH 7.0). The polarity of the R groups varies widely, from nonpolar and hydrophobic (water-insoluble) to highly polar and hydrophilic (water-soluble). The structures of the 20 common amino acids are shown in Figure 1.1, and some of their properties are listed in Table 1.1. Within each class there are gradations of polarity, size, and shape of the R groups. Nonpolar, Aliphatic R Groups The R groups in this class of amino acids are nonpolar and hydrophobic. The side chains of alanine, valine, leucine, and isoleucine tend to cluster together within proteins, stabilizing protein structure by means of hydrophobic interactions. Glycine has the simplest structure. Although it is formally nonpolar, its very small side chain makes no real contribution to hydrophobic interactions. Methionine, one of the two sulfur-containing amino acids, has a nonpolar thioether group in its side chain. Proline has an aliphatic side chain with a distinctive cyclic structure.
TABLE 1.1 Properties and Conventions Associated with the Common Amino Acids Found in Proteins

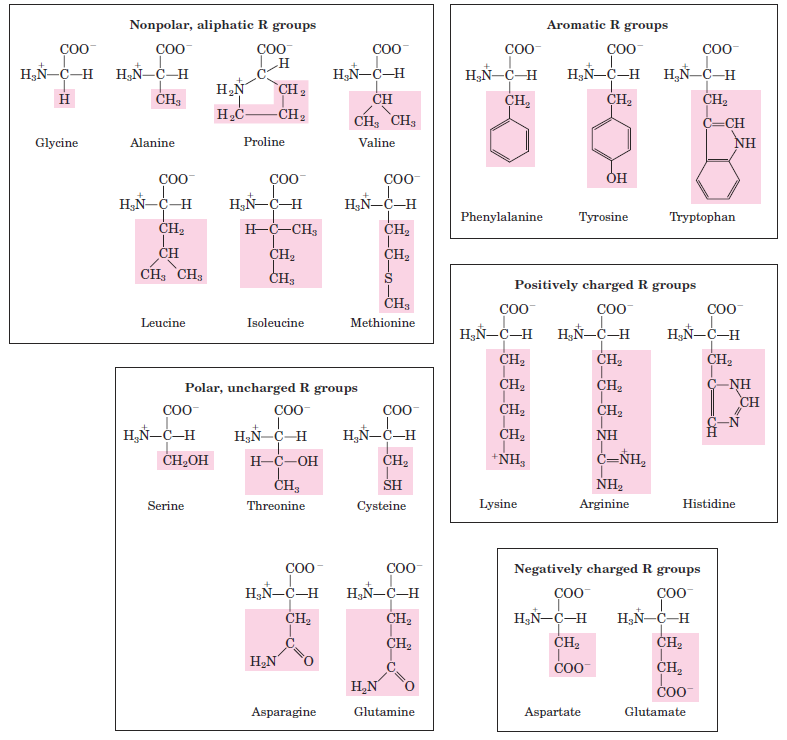
FIGURE 1.1 The 20 common amino acids of proteins. The structural formulas show the state of ionization that would predominate at pH 7.0. The unshaded portions are those common to all the amino acids; the portions shaded in red are the R groups. Although the R group of histidine is shown uncharged, its pKa (see Table 1.1) is such that a small but significant fraction of these groups are positively charged at pH 7.0.
The secondary amino (imino) group of proline residues is held in a rigid conformation that reduces the structural flexibility of polypeptide regions containing proline. Aromatic R Groups Phenylalanine, tyrosine, and tryptophan, with their aromatic side chains, are relatively nonpolar (hydrophobic). All can participate in hydrophobic interactions. The hydroxyl group of tyrosine can form hydrogen bonds, and it is an important functional group in some enzymes. Tyrosine and tryptophan are significantly more polar than phenylalanine, because of the tyrosine hydroxyl group and the nitrogen of the tryptophan indole ring. Tryptophan and tyrosine, and to a much lesser extent phenylalanine, absorb ultraviolet light . This accounts for the characteristic strong absorbance of light by most proteins at a wavelength of 280 nm, a property exploited by researchers in the characterization of proteins.
Polar, Uncharged R Groups The R groups of these amino acids are more soluble in water, or more hydrophilic, than those of the nonpolar amino acids, because they contain functional groups that form hydrogen bonds with water. This class of amino acids includes serine, threonine, cysteine, asparagine, and glutamine. The polarity of serine and threonine is contributed by their hydroxyl groups; that of cysteine by its sulfhydryl group; and that of asparagine and glutamine by their amide groups. Asparagine and glutamine are the amides of two other amino acids also found in proteins, aspartate and glutamate, respectively, to which asparagine and glutamine are easily hydrolyzed by acid or base. Cysteine is readily oxidized to form a covalently linked dimeric amino acid called cystine, in which two cysteine molecules or residues are joined by a disulfide bond (Fig. 1.3). The disulfide-linked residues are strongly hydrophobic (nonpolar). Disulfide bonds play a special role in the structures of many proteins by forming covalent links between parts of a protein molecule or between two different polypeptide chains. Positively Charged (Basic) R Groups The most hydrophilic R groups are those that are either positively or negatively charged. The amino acids in which the R groups have significant positive charge at pH 7.0 are lysine, which has a second primary amino group at the ε position
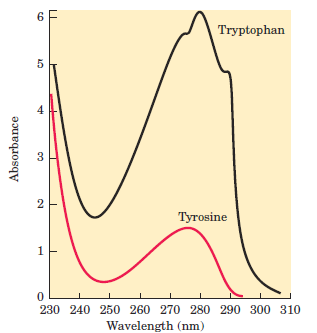
FIGURE 1.2Absorption of ultraviolet light by aromatic amino acids. Comparison of the light absorption spectra of the aromatic amino acids tryptophan and tyrosine at pH 6.0. The amino acids are present in equimolar amounts (10-3 M) under identical conditions. The measured absorbance of tryptophan is as much as four times that of tyrosine. Note that the maximum light absorption for both tryptophan and tyrosine occurs near a wavelength of 280 nm. Light absorption by the third aromatic amino acid, phenylalanine (not shown), generally contributes little to the spectroscopic properties of proteins.
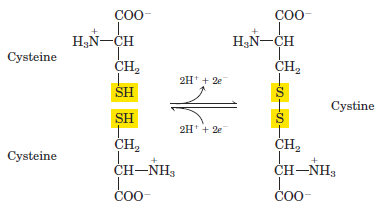
FIGURE 1.3 Reversible formation of a disulfide bond by the oxidation of two molecules of cysteine. Disulfide bonds between Cys residues stabilize the structures of many proteins.
on its aliphatic chain; arginine, which has a positively charged guanidino group; and histidine, which has an imidazole group. Histidine is the only common amino acid having an ionizable side chain with a pKa near neutrality. In many enzyme-catalyzed reactions, a His residue facilitates the reaction by serving as a proton donor/acceptor.
Negatively Charged (Acidic) R Groups The two amino acids having R groups with a net negative charge at pH 7.0 are aspartate and glutamate, each of which has a second carboxyl group.



|
|
|
|
تفوقت في الاختبار على الجميع.. فاكهة "خارقة" في عالم التغذية
|
|
|
|
|
|
|
أمين عام أوبك: النفط الخام والغاز الطبيعي "هبة من الله"
|
|
|
|
|
|
|
المجمع العلمي ينظّم ندوة حوارية حول مفهوم العولمة الرقمية في بابل
|
|
|