


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 30-5-2016
Date: 26-11-2018
Date: 4-12-2018
|
Group 17 :Physical properties and bonding considerations
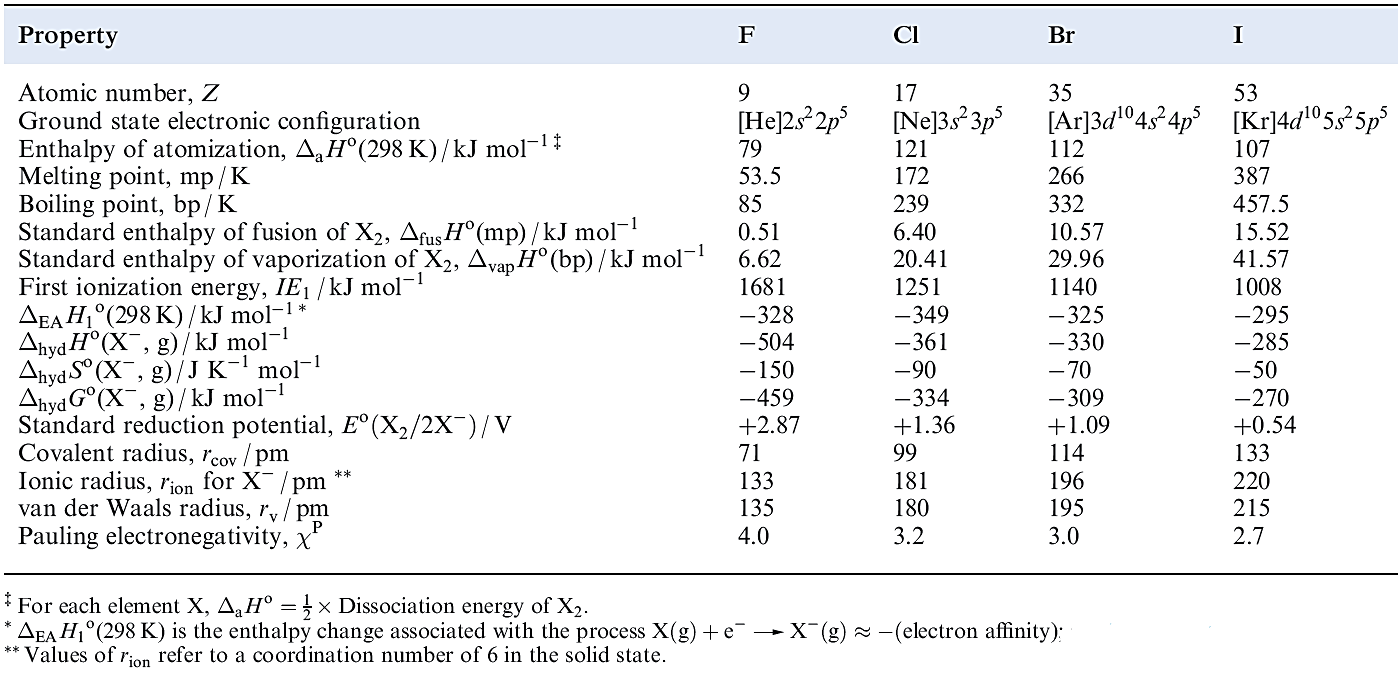
Table 1.1 lists selected physical properties of the group 17 elements (excluding astatine). Most of the differences between fluorine and the later halogens can be attributed to the:
The last factor is not a rigidly defined quantity. However, it is useful in rationalizing such observations as the anomalous physical properties of, for example, HF the strength of F-substituted carboxylic acids, the deactivating effect of the CF3 group in electrophilic aromatic substitutions, and the non-basic character of NF3 and (CF3(3N.
Fluorine forms no high oxidation state compounds (e.g. there are no analogues of HClO3 and Cl2O7). When F is attached to another atom, Y, the Y_F bond is usually stronger than the corresponding Y_Cl bond. If atom Y possesses no lone pairs, or has lone pairs but a large rcov, then the Y_F bond is much stronger than the corresponding Y_Cl bond. Consequences of the small size of the F atom are that high coordination numbers can be achieved in molecular fluorides YFn, and good overlap of atomic orbitals between Y and F leads to short, strong bonds, reinforced by ionic contributions when the difference in electronegativities of Y and F is large.

Fig. 1.1 The trend in X_X bond energies for the first four halogens.
The volatility of covalent F-containing compounds originates in the weakness of the intermolecular van der Waals or London dispersion forces. This, in turn, can be correlated with the low polarizability and small size of the F atom. The small ionic radius of F- leads to high coordination numbers in saline fluorides, high lattice energies and highly negative values of ΔfHo for these compounds, as well as a large negative standard enthalpy and entropy of hydration of the ion (Table 1.1).
Table 1.1 Some physical properties of fluorine, chlorine, bromine and iodine.
As expected, this is even more true in group 16. Table 1.1 lists values of the first ionization energies simply to show the expected decrease down the group. Although none of the halogens has yet been shown to form a discrete and stable monocation X+ , complexed or solvated I is established, e.g. in [I(py)2]+ (Figure 1.2), [Ph3PI] + and, apparently, in solutions obtained from reaction 1.1.
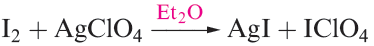 (1.1)
(1.1)
The corresponding Br- and Cl-containing species are less stable, though they are probably involved in aromatic bromination and chlorination reactions in aqueous media. The electron affinity of F is out of line with the trend observed for the later halogens (Table 1.1). Addition of an electron to the small F atom is accompanied by greater electron–electron repulsion than is the case for Cl, Br and I, and this probably explains why the process is less exothermic than might be expected on chemical grounds. As we consider the chemistry of the halogens, it will be clear that there is an increasing trend towards higher oxidation states down the group; this is well exemplified among the interhalogen compounds.

Fig. 1.2 (a) The structure of [I(py)2] + (determined by X-ray crystallography) from the salt [I(py)2][I3].2I2 [O. Hassel et al. (1961) Acta Chem. Scand., vol. 15, p. 407]; (b) A representation of the bonding in the cation. Colour code: I, gold; N, blue; C, grey.



|
|
|
|
تفوقت في الاختبار على الجميع.. فاكهة "خارقة" في عالم التغذية
|
|
|
|
|
|
|
أمين عام أوبك: النفط الخام والغاز الطبيعي "هبة من الله"
|
|
|
|
|
|
|
قسم شؤون المعارف ينظم دورة عن آليات عمل الفهارس الفنية للموسوعات والكتب لملاكاته
|
|
|