


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 30-3-2019
Date: 11-11-2018
Date: 25-11-2018
|
Gallium, indium and thallium
Since 1980, interest in organometallic compounds of Ga, In and Tl has grown, mainly because of their potential use as precursors to semiconducting materials such as GaAs and InP. Volatile compounds are sought that can be used in the growth of thin films by MOCVD (metal organic chemical vapour deposition) or MOVPE (metal organic vapour phase epitaxy) techniques. Precursors include appropriate Lewis base adducts of metal alkyls, e.g. Me3Ga.NMe3 and Me3In.PEt3. Reaction 1.1 is an example of the thermal decomposition of gaseous precursors
to form a semiconductor which can be deposited in thin films (see Box 18.4).
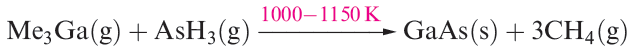 (1.1)
(1.1)
Gallium, indium and thallium trialkyls, R3M, can be made by use of Grignard reagents (reaction 1.2), RLi (equation 1.3) or R2Hg (equation 1.4), although a variation in strategy is usually needed to prepare triorganothallium derivatives (e.g. reaction 1.5) since R2TlX is favoured in reactions 1.2 or 1.3. The Grignard route is valuable for the synthesis of triaryl derivatives. A disadvantage of the Grignard route is that R3M.OEt2 may be the isolated product.
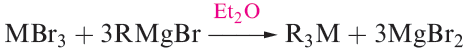 (1.2)
(1.2)
 (1.3)
(1.3)
 (1.4)
(1.4)
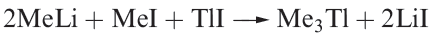 (1.5)
(1.5)
Trialkyls and triaryls of Ga, In and Tl are monomeric (trigonal planar metal centres) in solution and the gas phase. In the solid state, monomers are essentially present, but close intermolecular contacts are important in most structures. In trimethylindium, the formation of long In…..C interactions (Figure 18.10a) means that the structure can be described in terms of cyclic tetramers; further, each In centre forms an additional weak In…..C interaction (356pm) with the C atomof an adjacent tetramer to give an infinite network. The solid state structures of Me3Ga and Me3Tl resemble that of Me3In.
Within the planar Me3Ga and Me3Tl molecules, the average Ga_C and Tl_C bond distances are 196 and 230 pm, respectively. Within the tetrameric units, the Ga-----C and Tl-----C separations are 315 and 316pm, respectively. Intermolecular interactions are also observed in, for example, crystalline Ph3Ga, Ph3In and (PhCH2)3In. Figure 18.10b shows one molecule of (PhCH2(3In, but each In atom interacts weakly with carbon atoms of phenyl rings of adjacent molecules. Dimer formation is observed in Me2Ga)µ-C≡CPh(2GaMe2 (Figure 18.10c), and the same bonding description that we outlined for R2Al)PhC≡C(2AlR2 (18.9 and 18.10) is appropriate. Triorganogallium, indium and thallium compounds are airand moisture-sensitive. Hydrolysis initially yields the linear [R2M]+ ion (which can be further hydrolysed), in contrast to the inertness of R3B towards water and the formation of Al(OH)3) from R3Al. The [R2Tl]+ cation is also present in R2TlX (X = halide), and the ionic nature of this compound differs from the covalent character of R2MX for the earlier group 13 elements. Numerous adducts R3M.L (L= Lewis base) are known in which the metal centre is tetrahedrally sited, e.g. Me3Ga.NMe3, Me3 Ga.NCPh, Me3In.OEt2, Me3In.SMe2, Me3Tl.PMe3, [Me4Tl]- . In compound 1.1, donation of the lone pair comes from within the organic moiety; the GaC3-unit is planar since the ligand is not flexible enough for the usual tetrahedral geometry to be adopted.

(1.1)
Species of type [E2R4] (single E_E bond) and [E2R4]- (E_E bond order 1.5) can be prepared for Ga and In provided that R is especially bulky (e.g. R =)Me3Si)2CH, 2,4,6-iPr3C6H2), and reduction of [(2,4,6-iPr3C6H2)4Ga2] to [(2,4,6-iPr3C6H2(4Ga2]- is accompanied by a shortening of the Ga_Ga bond from 252 to 234 pm, consistent with an increase in bond order (1 to 1.5). By using even bulkier substituents, it is possible to prepare gallium(I) compounds, RGa (1.2) starting from gallium(I) iodide. No structural data are yet available for these monomers. However, 1.2 with R’ = H crystallizes as the weakly bound dimer 1.3, reverting to a monomer when dissolved in cyclohexane. The Ga_Ga bond in 1.3 is considered to possess a bond order of less than 1. Reduction of 1.3 by Na leads to Na2[RGaGaR], in which the dianion retains the trans-bent geometry of 1.2, and the Ga_Ga bond length is 235 pm. The salt Na2[RGaGaR] can also be prepared from the reaction of RGaCl2 and Na in Et2O, and it has been claimed that [RGaGaR]2- contains a gallium–gallium triple bond. However, recent results suggest that the Ga_Ga interaction in Na2[RGaGaR] is best described as consisting of a single bond, augmented by the weak interaction present in the precursor 1.2. Additionally, in the solid state, there are stabilizing interactions between the two Na ions and the Ga_Ga bond.
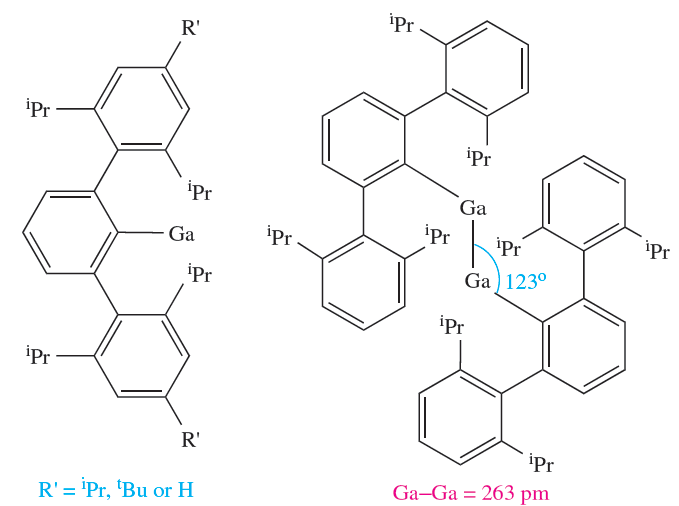
(1.2) (1.3)
The 2,6-dimesitylphenyl substituent is also extremely sterically demanding, and reduction of (2,6-Mes2C6H3)GaCl2 with Na yields Na2[)2,6-Mes2C6H3(3Ga3]; the [(2,6- Mes2C6H3)3Ga3]2- anion possesses the cyclic structure (1.4) and is a 2π-electron aromatic system.
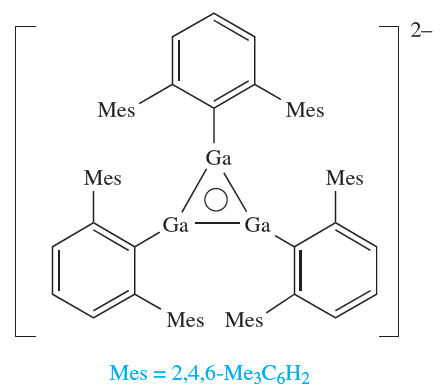
(1.4)
In equation 12.47, we illustrated the use of the metastable GaBr as a precursor to multinuclear Ga-containing species. Gallium(I) bromide has also been used as a precursor to a number of organogallium clusters. For example, one of the products of the reaction of GaBr with (Me3Si)3CLi in toluene at 195K is 1.5.
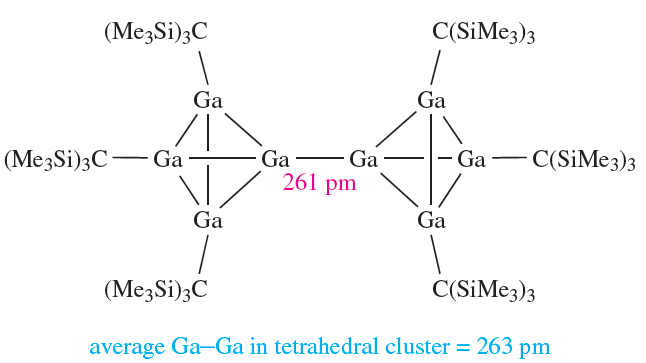
(1.5)
Worked example : Reactions of {(Me3Si)3C}4E4 (E = Ga or In)
The reaction of the tetrahedral cluster {(Me3Si)3C}4Ga4 with I2 in boiling hexane results in the formation of {(Me3Si)3CGaI}2 and {(Me3Si)3CGaI2}2. In each compound there is only one Ga environment. Suggest structures for these compounds and state the oxidation state of Ga in the starting material and products. The starting cluster is a gallium(I) compound:
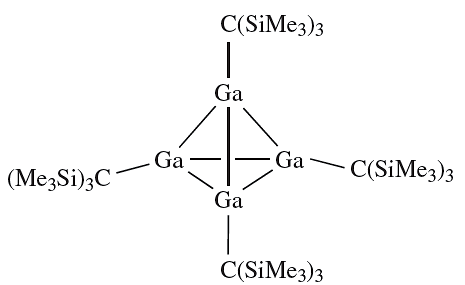
I2 oxidizes this compound and possible oxidation states are Ga(II) (e.g. in a compound of type R2Ga_GaR2) and Ga(III). {(Me3Si)3CGaI}2 is related to compounds of type R2Ga_GaR2; steric factors may contribute towards a nonplanar conformation:

Further oxidation by I2 results in the formation of the Ga(III) compound {(Me3Si)3CGaI2}2 and a structure consistent with equivalent Ga centres is:
Self-study exercises
1. The Br2 oxidation of {(Me3Si)3C}4In4 leads to the formation of the In(II) compound {(Me3Si)3C}4In4Br4 in which each In atom retains a tetrahedral environment. Suggest a structure for the product.
2. {(Me3Si)3CGaI}2 represents a Ga(II) compound of type R2Ga2I2. However, ‘Ga2I4’, which may appear to be a related compound, is ionic. Comment on this difference.
3. A staggered conformation is observed in the solid state for {(Me3Si)3CGaI}2. It has been suggested that a contributing factor may be hyperconjugation involving Ga_I bonding electrons. What acceptor orbital is available for hyperconjugation, and how does this interaction operate?
Cyclopentadienyl complexes illustrate the increase in stability of the M(I) oxidation state as group 13 is descended, a consequence of the thermodynamic 6s inert pair effect
Cyclopentadienyl derivatives of Ga(III) which have been prepared (equations 1.6 and 1.7) and structurally characterized include Cp3Ga and CpGaMe2.
 (1.6)
(1.6)
 (1.7)
(1.7)
The structure of CpGaMe2 resembles that of CpAlMe2 (Figure 18.9a), and Cp3Ga is monomeric with three η1-Cp groups bonded to trigonal planar Ga (Figure 1.1a).
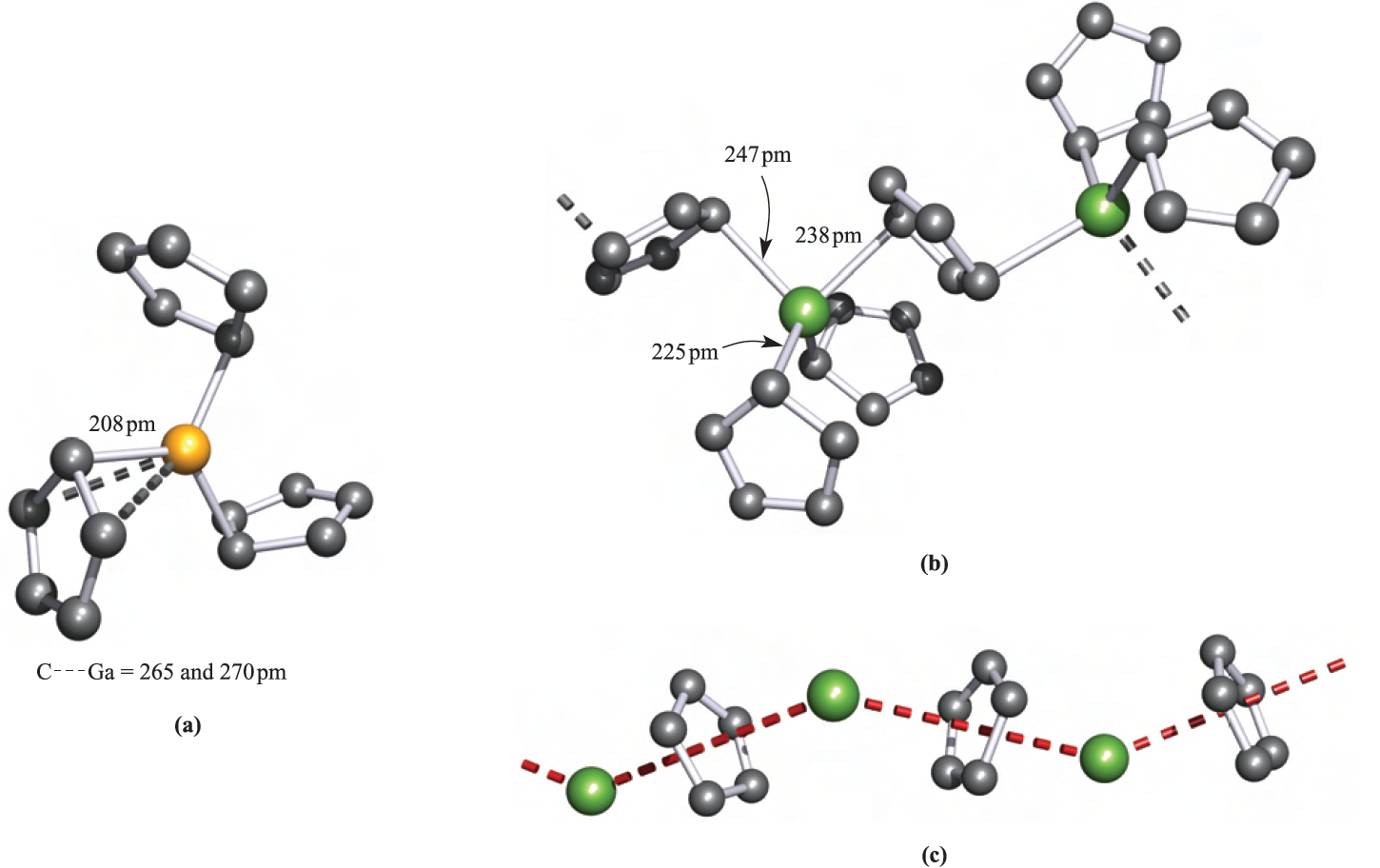
Fig. 1.1 The solid state structures (X-ray diffraction) of (a) monomeric )η1-Cp)3Ga [O.T. Beachley et al. (1985) Organometallics, vol. 4, p. 751], (b) polymeric Cp3In [F.W.B. Einstein et al. (1972) Inorg. Chem., vol. 11, p. 2832] and (c) polymeric CpIn [O.T. Beachley et al. (1988) Organometallics, vol. 7, p. 1051]; the zig-zag chain is emphasized by the red hashed line. Hydrogen atoms are omitted for clarity; colour code: Ga, yellow; In, green; C, grey.
InCl3, but is structurally different from Cp3Ga; the solid contains polymeric chains in which each In atom is distorted tetrahedral (Figure 1.1b). The reaction of (η5-C5Me5)3Ga with HBF4 results in the formation of [(C5Me5)2Ga]+[BF4]-. In solution, the C5Me5 groups are fluxional down to 203 K, but in the solid state the complex is a dimer (1.6) containing [(η1-C5Me5)(η3- C5Me5)Ga]+ ions. The structure of [(C5Me5)2Ga]+ contrasts with that of [(C5Me5)2Al]+, in which the C5-rings are coparallel.
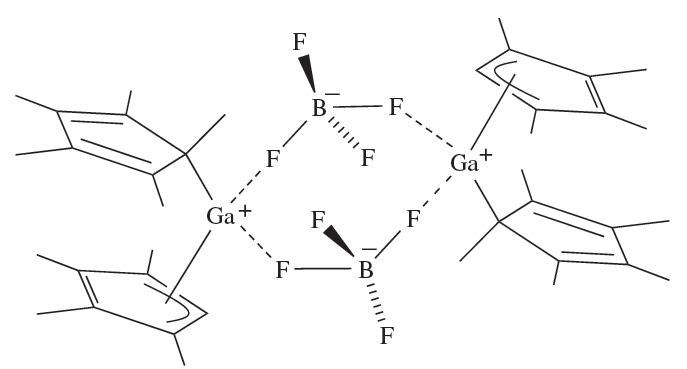
(1.6)
We saw earlier that gallium(I) halides can be used to synthesize ArGa compounds. Similarly, metastable solutions of GaCl have been used to prepare (C5Me5)Ga by reactions with (C5Me5)Li or (C5Me5)2Mg. An alternative route is the reductive dehalogenation of (C5Me5)GaI2 using potassium with ultrasonic activation. In the gas phase and in solution, (C5Me5)Ga is monomeric, but in the solid state, hexamers are present. On moving down group 13, the number of M(I) cyclopentadienyl derivatives increases, with a wide range being known for Tl(I). The condensation of In vapour (at 77 K) onto C5H6 gives CpIn, and CpTl is readily prepared by reaction 1.8.
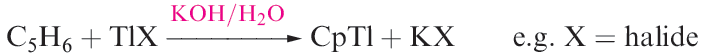 (1.8)
(1.8)
Both CpIn and CpTl are monomeric in the gas phase, but in the solid, they possess the polymeric chain structure shown in Figure 1.1c. The cyclopentadienyl derivatives(C5R5(M (M = In, Tl) are structurally diverse in the solid state, e.g. for R =PhCH2 and M = In or Tl, ‘quasi-dimers’ 1.7 are present (there may or may not be a meaningful metal– metal interaction), and (η5-C5Me5(In forms hexameric clusters.
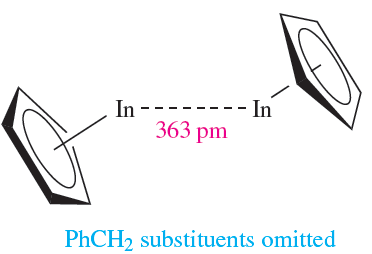
(1.7)
One use of CpTl is as a cyclopentadienyl transfer reagent to d-block metal ions, but it can also act as an acceptor of Cp-, reacting with Cp2Mg to give [Cp2Tl]-. This can be isolated as the salt [CpMgL][Cp2Tl] upon the addition of the chelating ligand L = Me2NCH2CH2NMeCH2CH2NMe2. The anion [Cp2Tl]- is isoelectronic with Cp2Sn and possesses a structure in which the η5-Cp rings are mutually tilted. The structure is as shown in Figure 1.2c for Cp2Si but with an angle α = 1570. Although this ring orientation implies the presence of a stereochemically active lone pair, it has been shown theoretically that there is only a small energy difference (3.5 kJmol-1) between this structure and one in which the η5-Cp rings are parallel (i.e. as in Figure 1.2a). We return to this scenario at the end of the next section.



|
|
|
|
تفوقت في الاختبار على الجميع.. فاكهة "خارقة" في عالم التغذية
|
|
|
|
|
|
|
أمين عام أوبك: النفط الخام والغاز الطبيعي "هبة من الله"
|
|
|
|
|
|
|
قسم شؤون المعارف ينظم دورة عن آليات عمل الفهارس الفنية للموسوعات والكتب لملاكاته
|
|
|