


 النبات
النبات
 الحيوان
الحيوان
 الأحياء المجهرية
الأحياء المجهرية
 علم الأمراض
علم الأمراض
 التقانة الإحيائية
التقانة الإحيائية
 التقنية الحيوية المكروبية
التقنية الحيوية المكروبية
 التقنية الحياتية النانوية
التقنية الحياتية النانوية
 علم الأجنة
علم الأجنة
 الأحياء الجزيئي
الأحياء الجزيئي
 علم وظائف الأعضاء
علم وظائف الأعضاء
 الغدد
الغدد
 المضادات الحيوية
المضادات الحيوية|
Read More
Date: 2-11-2015
Date: 19-10-2016
Date: 27-10-2016
|
Types of Respiration in Plants
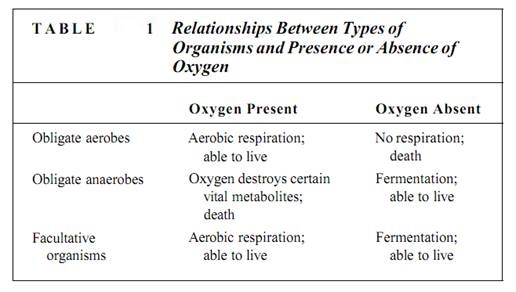
Cellular respiration falls into two categories: aerobic and anaerobic. Respiration that requires oxygen as the terminal electron acceptor is aerobic respiration. Under certain conditions, oxygen is not available and an alternative electron acceptor must be used. This is anaerobic respiration, respiration without oxygen, often called fermentation. Because animals and plants must have oxygen for their respiration, they are known as obligate or strict aerobes (Table 1). At the opposite extreme are certain bacteria called obligate anaerobes, which carry out anaerobic respiration exclusively; such bacteria are actually killed by oxygen. Many fungi and certain types of tissues in animals and some plants are facultatively aerobic (or facultatively anaerobic): If oxygen is present, they carry out aerobic respiration, but when oxygen is absent or insufficient, they switch to anaerobic respiration. Although many fungi, especially the yeasts, can live indefinitely anaerobically, plant and animal tissues can survive this way for only a short time. They must eventually obtain oxygen and switch back to aerobic respiration or they die.
ANAEROBIC RESPIRATION
Because glucose is broken down during anaerobic respiration, the metabolic pathway is given the name glycolysis (from Greek: glyky-sweet, and lysis-to break down). It is also called the Embden-Meyerhoff pathway in honor of the physiologists who first elucidated its steps. A comparison of Figures 1 shows that glycolysis and gtaconeogenesis are essentially the same pathway, with the reactions running in opposite directions. Although all intermediates are the same, as are many of the enzymes, the two processes use different enzymes at certain key steps. This allows a cell to regulate the two processes so that one is stopped while the other runs; it would be useless for both pathways to operate simultaneously within a single cell.
In glycolysis, ATP phosphorylates glucose to glucose-6-phosphate, which is then converted to fructose-6-phosphate. A second molecule of ATP then phosphorylates this to fructose-1,6-bisphosphate, which breaks down into 3-phosphoglyceraldehyde and dihydroxyacetone phosphate. The latter can be converted to 3-phosphoglyceraldehyde, of course, which can be oxidized to 1,3-diphosphoglycerate. During this oxidation step electrons are transferred from a carbon of 3-phosphoglyceraldehyde to NAD+, converting it to ATP and changing the 1,3-diphosphoglycerate into 3-phosphoglycerate, a process called substrate level phosphorylation .
The enzyme is phosphoglycerate kinase; the kinases constitute a large group of enzymes that remove phosphate groups from substrates. Phosphorylases are just the opposite, adding phosphates to substrates.
Although this is basically a reversal of the stroma reactions, ribulose-l,5-bisphosphate does not occur next as one might expect. Instead, 3-phosphoglycerate is converted first to 2-phosphoglycerate and then to phosphoenolpyruvate (PEP), the same metabolite that is the carbon dioxide acceptor in C4 metabolism. PEP is also energetic enough that an enzyme can transfer its phosphate group onto ADP to make ATP; de-phosphorylation causes PEP to become pyruvate.

FIGURE 1:Glycolysis, also called the Embden-Meyerhoff pathway, constitutes the major portion of anaerobic respiration and is also the first part of aerobic respiration.
If no oxygen is present, anaerobic respiration has now removed all the energy possible. From each molecule of glucose, four ATPs were generated and two were consumed, so there is a net production of two ATPs. Occasionally the cell can start with glucoses-phosphate and use one less ATP to get started. The ATP generated in anaerobic respiration is used for other metabolic reactions such as protein synthesis, nucleic acid replication, microtubule assembly, and ion transport. Indeed, these metabolic pathways are the reasons for respiration, and the ADP they generate migrates back to the sites of glycolysis and is re-phosphorylated to ATP.
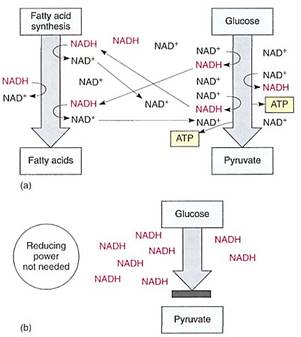
FIGURE 2 (a) If a cell needs reducing power, as for the synthesis of fatty acids, those reactions are actually electron-accepting reactions and they regenerate NAD+, allowing the continued production of AIP by glycolysis. (b) If reducing power is not needed, NAD+/NADH accumulates as NADH. Without NAD+, glycolysis stops: 3-phosphoglyceraldehyde cannot be oxidized to 1,3-diphosphoglycerate, and no ATP can be formed.
The reduction of NAD4" to NADH during glycolysis is a problem. If the cell needs reducing power, the NADH can be used and it regenerates the NAD+ necessary to keep glycolysis running. For example, roots absorb nitrates and sulfates that must be reduced, and during the synthesis of fatty acids, large amounts of reducing power are needed. Many of these reductions can be carried out with this extra NADH (Fig. 2). But usually a cell does not need as much reducing power as is produced during respiratory ATP production; consequently, NADH accumulates, all the NAD+ is consumed, and glycolysis stops for lack of NAD+. Without further glycolysis, no ATP would form and death would result. The big problem is converting NADH back to NAD+ by dumping its electrons. In animal tissues under anaerobic conditions, the electron acceptor is pyruvate: NADH reacts with it to £m lactate, the anion of lactic acid (Fig. 3). In plants and fungi, pyruvate is first converted to acetaldehyde and then NADH reacts with that, forming ethanol (ethyl alcohol; fig. 4a). Anaerobic conditions occur in plants and fungi growing in mud beneath stagnant water, especially in swamps and marshes (Fig. 4b). Rice seeds germinate and grow anaerobically until the shoots reach oxygenated water (Fig. 5).
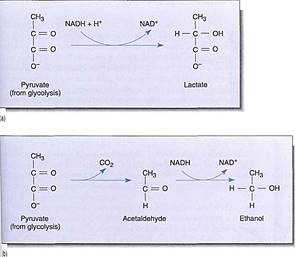
FIGURE 3 :(a) When we exercise slowly enough for blood circulation to keep up, all our muscular activity is aerobic. But with rapid, intense, and prolonged activity, blood does not carry oxygen to the muscles rapidly enough and lactic acid fermentation begins. Lactate accumulation causes cramps and muscle pain. (b) Alcoholic fermentation involves the conversion of pyruvate to acetaldehyde before reduction by NADH + H+.

FIGURE 4:(a) In wine-making, yeast cells (Saccharomyces) ferment the glucose present in grapes and excrete the wasle product, ethanol. Naturally fermented wine has a maximum alcohol content of 14%; at that point, the waste product kills the yeast. Beer has a lower alcohol content, 5.5%, not because its yeast is more sensitive to the ethanol but because it is the legal limit. Fermentation must be stopped artificially, usually by heating the beer to kill the yeast. Alcoholic beverages with alcohol content higher than 14% have extra alcohol added or part of their water removed (X 430). (b) The leaves and upright stems of water lily (Nuphar) exist in a highly oxygenated aerobic environment, but the rhizomes and roots exist in an anaerobic muck. Aerenchyma tissues in the stems permit the diffusion of some oxygen from leaves to rhizomes and roots, but some anaerobic respiration may be occurring. (b, © Butch Gemin)
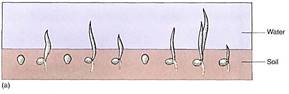
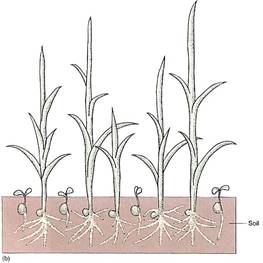

FIGURE 5: (a) Even under flooded, anaerobic conditions, rice seeds germinate and begin to grow because their embryos carry out facultative anaerobic respiration. Other seeds (round) remain dormant because they are incapable of fermentation; being dormant, they have a low rate of metabolism and the small amount of oxygen present may keep them from dying immediately. (b) When flooding subsides, oxygen is available and non-rice seeds germinate, but they are shaded by rice plants, and the soil is already filled with rice roots. The rice plants have a tremendous advantage. (c) Rice seedlings, still flooded. (d) Fields of rice. The lines are the dikes used to flood the fields for irrigation. (candd, Courtesy of M. Woods/The Rice Council)
Ethanol and lactate are not especially good solutions to the problem of NADH accumulation. The pyruvate consumed is always present in adequate amounts, of course, because glycolysis itself produces it, but it is not really "cheap" because many of its bonding orbitals have high-energy electrons. Furthermore, pyruvate could be used as a monomer for many types of synthesis. Lack of oxygen forces a cell to use a very valuable molecule as an electron dumping ground. Even worse, the products, either lactate or ethanol, are toxic. If they accumulate in the tissues or environment, they damage or even kill the cells producing them (Table 2).
Considering the negative aspects of anaerobic respiration, how could natural selection have produced something so inefficient? At the time life arose and respiratory pathways were evolving, Earth's atmosphere contained reduced, hydrogen-rich compounds but no oxygen . Consequently, pyruvate and acetaldehyde had to be used as electron acceptors. Millions of years later, photosynthesis based on chlorophyll a evolved, and oxygen was released to the environment as a result of the water-splitting involved. after free oxygen became relatively abundant, the mutations leading to aerobic respiration began to be selectively advantageous.
Even in the presence of an oxygen-rich atmosphere and aerobic respiration, retaining the capacity for anaerobic respiration still has some selective advantage. Although the method is far from ideal, it does allow certain organisms to survive in particular environments. For example, because rice seedlings are capable of anaerobic respiration, they can germinate and establish themselves during times of floods when other plants or seedlings are suffocating (Fig. 5). In the absence of oxygen, the alternative to anaerobic respiration is death (see Table 1). Although this metabolism is expensive for the rice, by the time the flooding subsides, rice plants are already well-rooted, have several leaves, and outcompete other species whose seeds are just beginning to germinate.

AEROBIC RESPIRATION
With oxygen present, the problems of using pyruvate or acetaldehyde as an electron acceptor are eliminated; oxygen is absorbed and acts as the terminal electron acceptor. oxygen is inexpensive because it is absorbed and distributed by molecular diffusion, which requires neither active transport nor ATP consumption. Oxygen is abundant in most situations, and the product of reduction is water, which is not only nontoxic but actually beneficial. Aerobic respiration consists of three parts: (1) glycolysis, (2) the citric acid cycle, and (3) oxidative phosphorylation in an electron transport chain.
Glycolysis. The initial steps of aerobic and anaerobic respiration are identical: glycolysis by the Embden-Meyerhoff pathway to pyruvate . This produces ATP and NADH just as before, but with oxygen present the NADH migrates to electron carriers that oxidize it back to NAD+, permitting glycolysis to continue. Glycolysis occurs in the cytosol.
The Citric Acid Cycle. Because pyruvate is not needed as an electron acceptor, it can be used in a number of metabolic pathways. One of the main pathways takes advantage of the large amount of energy remaining in pyruvate and, rather than using it for its structure, breaks it down and generates more ATP. This pathway is called the citric acid cycle, the Krebs cycle, or the tricarboxylic acid cycle. These names reflect different facts about the cycle One of the intermediates is citrate, the anion of citric acid. Much of the pioneering work on this metabolism was carried out by Hans Krebs. Finally, several of the intermediates are tricarboxylic acids—that is, each has three carboxyl (—COOH) groups.

FIGURE 6:The process by which pyruvate is attached to CoA releases a carbon dioxide molecule and forms NADH. This step does not occur during anaerobic respiration; not only would it use up pyruvate needed as an electron acceptor, but it would generate even more NADH. Both of those results would be detrimental under anaerobic conditions, but both are beneficial when oxygen is present.
In the citric acid cycle, pyruvate is transported from the cytosol, where glycolysis occurs, across the mitochondrial membranes to the mitochondrial matrix. There it is oxidized and de-carboxylated: As the electrons are transferred to NAD+, the bonding orbitals holding the last COO" rearrange and CO2 is liberated. Carbon dioxide and NADH are produced, along with a two-carbon fragment called acetyl (Fig. 6). The carbon dioxide ad NADH remain free in the matrix solution, but the acetyl becomes attached to a carrier molecule, coenzyme A (CoA), the resulting combination being acetyl CoA. Like pyruvate, acetyl CoA can be used in many synthetic pathways, but here we are interested in its entry into the citric acid cycle (Fig. 7) by transfer of the acetyl group to an acceptor molecule, oxaloacetate, a compound with four carbons. The oxaloacetate is converted to a six-carbon compound, citrate, which is then rearranged to cis-aconitate, which in turn is transformed to isocitrate (Fig. 7, steps A, B, C, D).

FIGURE 7:The steps of the citric acid cycle. This cycle is an important part of aerobic respiration, even though it does not consume oxygen and generates only one molecule of ATP directly. DH = dehydrogenation (oxidation); DC = decarboxylation.
In the next step, one of the carbons of isocitrate is oxidized by passing electrons onto NAD+, creating NADH. The oxidized carbon is liberated as carbon dioxide, leaving alpha-ketoglutarate, which has only live carbons (step E). This too is oxidized by NAD+, liberating another carbon dioxide, and the four-carbon remnant becomes attached to a new molecule of CoA in the process, forming succinyl CoA (step F). The energy released by the breakdown into free CoA and free succinate can power phosphorylation of ADP to ATP (step G). The succinate still contains considerable energy and is oxidized to fumarate as electrons and protons are passed to flavin adenine dinucleotide (FAD), reducing it to FADH2 (Fig. 8).
Although the molecule becomes oxidized by this step, no carbon dioxide is lost; the fumarate is also a four-carbon compound. It reacts with a water molecule and becomes malate, which passes a final set of electrons onto NAD+ and is transformed into the original acceptor molecule, oxaloacetate (steps H, I, J).

FIGURE 8:Flavin adenine dinucleotide (FAD) is a large organic electron carrier similar to NAD+. When it is reduced by two electrons, it picks up two protons, becoming FADH2. Unlike NADH + H+, both protons are covalently attached to FADH2.
It was mentioned above that the benefit of the citric acid cycle is the generation of more ATP, yet at only one step has ATP been produced. Instead, there are four steps in which more NAD+ is reduced to NADH and one in which FAD is reduced to FADH2. Excess NADH and the related deficiency of NAD+ were seen to be problems in anaerobic respiration, so the citric acid cycle at first seems to be a real contradiction. But in the next step, the electron transport chain, the energy in NADH and FADH2 drives the synthesis of ATP, and NADH is simultaneously oxidized back to NAD+.
The Mitochondrial Electron Transport Chain: Chemiosmotic Phosphorylation. The mitochondrial inner membrane, like the chloroplast inner membrane, contains sets of com-pounds capable of carrying electrons (Fig. 9). Although many of the actual carriers differ in the two organelles, the principles of electron transport are the same The carriers that react with NADH or oxygen are placed precisely and asymmetrically in the cristae membranes, both on the matrix side only (Fig. 10). At present, the exact order of carriers in plant mitochondria is not known, and numerous types exist. Only the most well-understood are presented below.
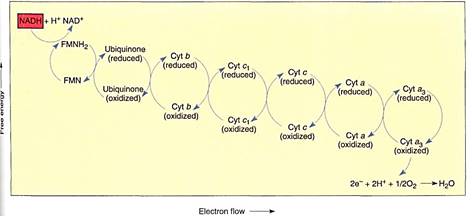
FIGURE 9:Membrane-bound electron carriers of the mitochondrial electron transport chain. As in chloroplasts, their positions and movements are important (see Fig. 10). Electrons are brought to membrane-bound carriers by mobile carriers such as NADH and FADH2, which are produced in the matrix.
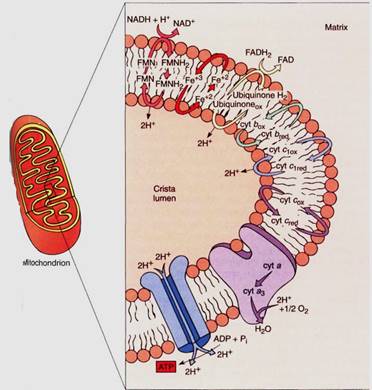
FIGURE 10:Only the important components of the mitochondrial electron transport chain are shown in this diagram—the steps that transport protons from the matrix to the crista lumen, establishing a proton/hydroxy1 chemiosmotic gradient, just as in chloroplasts. The formation of water contributes to the gradient because protons are absorbed from the matrix but not from the cristae. FMN is flavin mononucleotide, an electron carrier similar in structure to FAD.
NADH diffuses to the membrane and passes electrons to a protein that has flavin mononucleotide (FMN) bound to it as a cofactor. The FMN is reduced and the NADH simultaneously oxidized to NAD+, which can migrate back to the site of the citric acid cycle. The reduced FMN (FMNH2) passes the electrons to a set of iron- and sulfur-containing, proteinaceous electron carriers, which transfer the electrons to one or several quinones, one of which is ubiquinone (also called coenzyme Q; Fig. 11). From the pool of quinones, electrons are transferred to cytochrome b. The next carriers in sequence are more quinones, then cytochrome c1, cytochrome c, cytochrome a, and cytochrome a3 Cytochromes a and a3 are part of a large enzyme complex known as cytochrome oxidase, which contains several proteins and two copper ions that mediate the transfer of electrons from the iron in cytochrome to oxygen. As oxygen is reduced, it picks up two protons and becomes water.

FIGURE 11:Ubiquinone (coenzyme Q) is a quinone that can carry two electrons and two protons simultaneously.
If this electron transfer were the only thing to happen in the electron transport chain, it would be better than anaerobic respiration because the cell could avoid the waste of pyruvate and the synthesis of the toxic lactate or ethanol, but much more is accomplished. Mitochondria also use chemiosmotic phosphorylation. As with chloroplasts, the fate of the protons released and absorbed during electron transport is important. In mitochondria, when NADH reacts with FMN, two protons are transferred as well as electrons. One proton comes from the NADH and the other from the water, both on the matrix side of the membrane. When the FMNH2 reduces any quinone, the two protons are released on the other side of the membrane into the lumen of the crista. This step of electron transport acts as a proton pump; a large concentration of protons begins to build up in the lumen while there is a lack of protons in the matrix. Ubiquinone and the other quinones just before cytochrome c act in a similar manner, pumping protons out every time they carry electrons. Also, as oxygen receives electrons from the electron transport chain, it forms water by picking up two protons from the matrix, decreasing the proton concentration. In a short time, a proton concentration gradient develops which is strong enough to cause the protons to migrate back into the matrix.
The flow of protons from the crista lumen to the mitochondrial matrix can be used to synthesize ATP. As in chloroplasts, ATP synthetase channels in the membrane allow the flow of protons to force a phosphate group onto ADP, creating ATP. This is a chemiosmotic phosphorylation, and because of it, the NADH is an excellent if indirect source of AIP rather than a problem. The electrons that each molecule of NADH contributes to the mitochondrial electron transport chain provide enough power to create three ATPS.
The FADH2 also passes its electrons to the mitochondrial electron transport chain, but it reacts with ubiquinone instead of FMN, so the first step in proton pumping does not use these electrons. Therefore the FADH2 contributes less to the proton gradient than does NADH, providing enough power for the production of two ATPs rather than three.
The NADH Shuttle. NADH produced by glycolysis cannot cross the mitochondrial inner membrane and donate electrons directly to the electron transport chain because the inner membrane is impermeable to such large molecules. Instead, a series of chemical reactions carries (shuttles) reducing power across the membrane (Fig. 12). Several shuttle mechanisms occur. In the malate-aspartate shuttle, NADH in the cytosol powers the conversion of oxaloacetate to malate, which crosses to the mitochondrial matrix and powers the formation of a new molecule of NADH. Malate is converted to aspartate and transported back out of the mitochondrion, where it is converted to oxaloacetate again and can repeat the cycle. In this shuttle, each cytosolic NADH drives the formation of a matrix NADH and Ute consequent oxidative phosphorylation of three ADPs to three ATPS.
In a second type of shuttle, the glycerol phosphate shuttle, cytosolic NADH reduces dihydroxyacetone phosphate to glycerol phosphate, which is transported across the inner membrane to the matrix. There it converts back to dihydroxyacetone phosphate, reducing FAD to FADH2 in the process. Each cytosolic NADH results in the formation of one matrix FAEH2, which can drive the formation of only two ATPs; not as much energy is conserved in this shuttle.
In at least some plants, NADH can cross the outer mitochondrial membrane and react directly with ubiquinone at the outer surface of the inner membrane. A shuttle mechanism is not necessary, but the step of proton pumping by FMN is bypassed, decreasing the amount of ATP that can be generated.
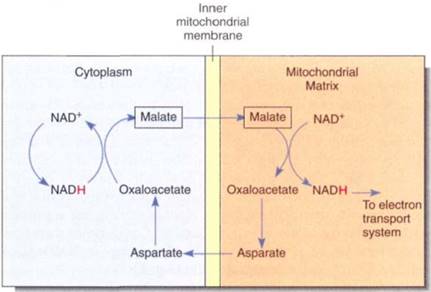
FIGURE 11.15:The malate-aspartate shuttle allows reducing power to be transferred into mitochondria when NADH itself cannot cross the mitochondrial membranes.
HEAT-GENERATING RESPIRATION
During glycolysis, the citric acid cycle, and mitochondrial electron transport, small amounts of energy are lost in each step even though a great deal of energy is conserved by the synthesis of ATP . The total chemical energy of all products (ATP, carbon dioxide, and water) is less than that of all reactants (gluclose-6-phosphate and oxygen); the difference is "lost" as heat and increased entropy (disorder). For example, compost piles become warm because of the heat loss during the respiration of the fungi and bacteria that decompose the compost. Heat loss is usually an inefficient aspect of respiration; in most cases, it would be selectively advantageous for a plant to produce more ATP from each molecule of glucose respired and lose less heat to the environment.
The heat "lost" during respiration by warm-blooded mammals is vitally necessary to maintain body temperature, and when we are chilled we must generate even greater amounts of heat by shivering: Our muscles contract and relax rapidly, breaking down large amounts of ATP and releasing the stored energy as heat.
Some plants also generate large amounts of heat. In the voodoo lily (Sauromatum guttatum), parts of the inflorescence become much warmer than the surrounding air, causing amines and other chemicals to vaporize and diffuse away as chemical attractants for pollinators. Skunk cabbage (Symplocarpusjoetidus) often begins floral development while covered with snow; it melts the snow cover and exposes its flowers by generating large quantities of heat (Fig. 13). These and other plants are able to produce heat much more efficiently than humans do; they have alternative electron carriers that apparently do not pump protons during electron transport in mitochondria. Consequently there is no proton gradient and no chemiosmotic production of ATP. The energy in NADH is converted entirely to heat, and the tissues become quite warm (Fig. 14).

FIGURE 13:By generating heat internally, skunk cabbage (Symplocarpusjoetidus) maintains a high enough temperature to carry out active metabolism even when covered by snow. When it is ready to emerge, it produces even more heat and melts the snow, revealing the inflorescence to pollinators. (L L Rue III/Earth Sciences)
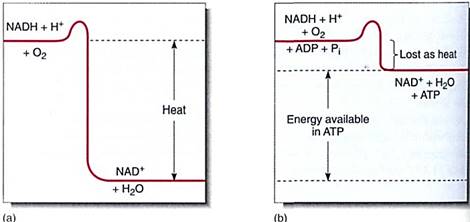
FIGURE 14 :If NADH breaks down to NAD+, a large amount of energy is liberated. (a) If this powerful exergonic reaction is not coupled to any endergonic reaction, all the energy is converted to heat; (b) When coupled to the endergonic synthesis of ATP from ADP, most of the energy is conserved and little is converted to heat.
In ordinary mitochondria, cyanide (CN-), azide (N3-), and carbon monoxide (CO) interfere with the last electron carrier, cytochrome oxidase. When they are present, electrons cannot pass from cytochrome c to oxygen; without electron flow and consequent ATP generation, both animals and plants die unless they are capable of anaerobic respiration. In plants that generate heat, the alternative electron carriers do not interact with cyanide, azide, or carbon monoxide, and heat is generated even if these chemicals are present. Heat-generating respiration can thus be studied by poisoning normal aerobic respiration with cyanide; consequently, heat-generating respiration is usually called cyanide-resistant respiration. A better name is thermogenic respiration.
The term "cyanide-resistant respiration" is somewhat misleading because it suggests that plants can be immune to cyanide poisoning. The analogy that because anaerobic respiration allows cells to survive in anaerobic conditions, cyanide-resistant respiration must allow cells to survive in the presence of cyanide is incorrect. Plants never encounter high concentrations of cyanide in natural conditions; if they did they would be killed because aerobic ATP generation is blocked. "Thermogenic respiration" and "heat-generating respiration" are less confusing terms.
Many aspects of thermogenic respiration are still completely unknown. We do not know if it occurs only in specialized mitochondria or if a single mitochondrion can have both types of electron carriers. Although we know that plants turn this respiration on and off at specific times, we do not know what the controlling mechanism is. We are not certain how widespread thermogenic respiration is; it has been found in many plant tissues as well as several algae, fungi, and bacteria.
PENTOSE PHOSPHATE PATHWAY
The pentose phosphate pathway is so named because it involves several intermediates that are phosphorylated five-carbon sugars (pentoses). It is usually included in discussions of respiration because it begins with glucose-6-phosphate, gives off carbon dioxide, and involves oxidations that produce NADPH (Fig. 15). However, its importance as a source of respiratory energy is much less significant than its role as a synthetic pathway. The pentose phosphate pathway transforms glucose into four-carbon sugars (erythrose) and five-carbon sugars (ribose) that are essential monomers in many metabolic pathways. The ribose-5-phosphate produced can be shunted into nucleic acid metabolism, forming the baas of RNA (ribonucleic acid) and DNA (deoxyribonucleic acid) monomers, the nucleotides. In meristematic cells, large amounts of DNA must be synthesized during the S-phase of a short cell cycle; the pentose phosphate pathway is an extremely important part of the metabolism of these cells.

FIGURE 15:The pentose phosphate pathway, showing all intermediates. If ribose-5-phosphate is drawn off into nucleic acid metabolism, the pentose phosphate pathway is shifted in favor of ribose production. But if erythrose-4-phosphate is diverted to lignin metabolism, the pentose phosphate pathway reaction equilibrium are shifted toward erythrose production.
The four-carbon sugar erythrose-4-phosphate is the starting material in the synthesis of many compounds. Two important types are lignin and anthocyanin pigments. Tissues such as wood, fibers, and sclereids deposit large amounts of lignin into their secondary walls during development, and erythrose-4-phosphate is in great demand. During differentiation, these cells use the pentose phosphate pathway but pull out erythrose-4-phosphate, not ribose-5-phosphate. Flower petals and brightly colored fruits divert erythrose-
4-phosphate from the pentose phosphate pathway to anthocyanin production while synthesizing their pigments. The pentose phosphate pathway also occurs in plastids, where it supplies erythrose-4-phosphate for synthesis of amino acids such as tyrosine, phenylalanine, and tryptophan.
In addition to these four-carbon and five-carbon sugars, the pentose phosphate pathway also produces NADPH. Although many anabolic reductions in the cytoplasm use NADH rather than NADPH, the reduction of nitrate (NO3") to amino acids (NH3+) can be accomplished only by NADPH, which can be generated by the pentose phosphate pathway.
Many of the reactants in the pentose phosphate pathway are the same as those in glycolysis, and both pathways occur in the cytosol. They are best understood as interconnected and simultaneous pathways. In meristematic cells the pentose phosphate pathway is active and shifted in favor of ribose production. In wood cells it is also active but produces erythrose. In other cells it may be much less active and glycolysis may dominate, producing NADH that then powers ATP production in mitochondria. Imagine a cell as it is produced in the vascular cambium (meristematic, needs nucleic acids), then differentiates into lignified wood parenchyma (needs lignin), which, after the wall is mature, takes on the role of storing starch during the summer and releasing it in the spring (Fig. 16). Energy metabolism is adjusted at each stage in a major way, and smaller changes in the rates of reactions may occur on a day-to-day basis.
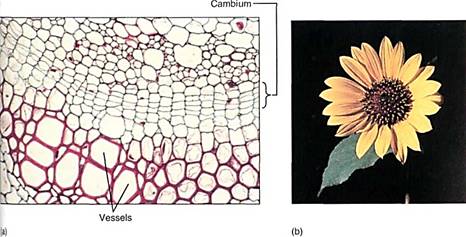
FIGURE 16 :(a) At the cambium, the pentose phosphate pathway may be producing ribose-5-phosphate, but in te differentiating vessels it is producing erythrose-4-phosphate. In all cells, glycolysis and the rest of aerobic respiration are also occurring simultaneously with the pentose phosphate pathway (X 400). (b) In petals, erythrose-4-phosphate, used in the production of pigments, is produced by the pentose phosphate pathway.
RESPIRATION OF LIPIDS
Some tissues, especially oily seeds and dormant apical meristems, store large amounts of lipid, usually as triglycerides or phospholipids. During germination or release form dormancy, lipids undergo catabolic metabolism in which they are broken down into glycerol three fatty acids (triglycerides) or glycerol phosphate and two fatty acids (phospholipids). Fatty acids are then further broken down into two-carbon units—acetyl CoA—by a process called beta-oxidation in either cytosol or microbodies called glyoxisomes. For example, an 18-carbon fatty acid would be converted into nine acetyl CoA units. As each acetyl CoA is formed, one FAD is reduced to FADH2 and one NAD+ is reduced to NADH, both of which can carry electrons to mitochondria and drive production of ATP by means of the electron transport chain (Fig. 17). Acetyl CoA may be used for synthesis of carbohydrates and other compounds or may enter the citric acid cycle and be further respired.

FIGURE 17:During the respiration of lipids, fatty acids are separated from glycerol and undergo beta-oxidation to acetyl CoA units, producing FADH2 and NADH. In germinating seeds, much of the acetyl CoA is converted to glucose and fructose by gluconeogenesis and then is polymerized into sucrose. The sucrose is transported by phloem from cotyledons to embryo meristems, where it is used in construction or respired for energy.
PHOTORESPIRATION
Photorespiration occurs only when RuBP carboxylase adds oxygen rather than carbon dioxide to ribulose-l,5-bisphosphate, resulting in one molecule of 3-phosphoglycerate and one of phosphoglycolate . Phosphoglycolate is dephosphorylated to glycolate, which is then transported to microbodies called peroxisomes. Glycolate can be converted to glycine in the peroxisomes, and the glycine may be transferred to mitochondria, where it is respired to carbon dioxide and water with no conservation of energy in either ATP or NADH; all energy is wasted. In some cases, some of the glycine can be converted to serine; both are useful amino acids. Other mechanisms also produce these amino acids, however, and direct measurements show that for many C3 species, photorespiration wastes as much as 30% of the energy trapped by photosynthesis.



|
|
|
|
دراسة يابانية لتقليل مخاطر أمراض المواليد منخفضي الوزن
|
|
|
|
|
|
|
اكتشاف أكبر مرجان في العالم قبالة سواحل جزر سليمان
|
|
|
|
|
|
|
المجمع العلمي ينظّم ندوة حوارية حول مفهوم العولمة الرقمية في بابل
|
|
|