


 النبات
النبات
 الحيوان
الحيوان
 الأحياء المجهرية
الأحياء المجهرية
 علم الأمراض
علم الأمراض
 التقانة الإحيائية
التقانة الإحيائية
 التقنية الحيوية المكروبية
التقنية الحيوية المكروبية
 التقنية الحياتية النانوية
التقنية الحياتية النانوية
 علم الأجنة
علم الأجنة
 الأحياء الجزيئي
الأحياء الجزيئي
 علم وظائف الأعضاء
علم وظائف الأعضاء
 الغدد
الغدد
 المضادات الحيوية
المضادات الحيوية|
Read More
Date: 25-12-2015
Date: 17-6-2021
Date: 28-4-2016
|
EGTA (ethyleneglycol bis (b-aminoethyl ether)-N,N,N′,N′-tetraacetic acid(
EGTA is a chelating agent widely used to control the concentration of Ca2+ cations in biological solutions (see also -Tetraacetic Acid)). Its structure is given in Figure 1. EGTA binds Ca2+3× 105-fold more strongly than Mg2+, which makes it useful to control Ca2+ levels in the presence of physiological concentrations of Mg2+ (1-3). The binding of cations by EGTA is pH-dependent; at low pH the carboxylate moieties will be protonated, dramatically lowering the cation affinity. The high selectivity of EGTA for Ca2+ over Mg2+ is readily explained by the structure of their respective complexes (Fig. 2, see top of next page) (4, 5). While EGTA fully wraps around Ca2+ in a very stable octadentate complex, it cannot similarly satisfy the more stringent binding requirements of the smaller Mg2+. The preference of Mg2+ for oxygen ligands over nitrogen ligands is so great that it binds to EGTA via the carboxylate groups only. The remaining positions in the ligand shell are filled by water. Although these complexes can be crystallized and their structure determined, they are very dynamic in solution. NMR analysis of the Ca2+-EGTA complex indicates that the carboxylate moieties interchange positions rapidly on the NMR time scale, and inversion at the nitrogen atoms is observed at elevated temperatures (5), which further indicates that the complex is very dynamic. The great stability of the Ca2+-EGTA complex comes from the fact that all ligands are provided by a single molecule, specifically, the stability constants have a very high entropic contribution.
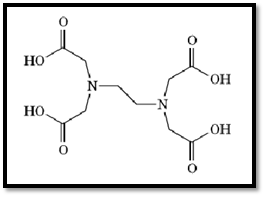
Figure 1. Molecular structure of EGTA.
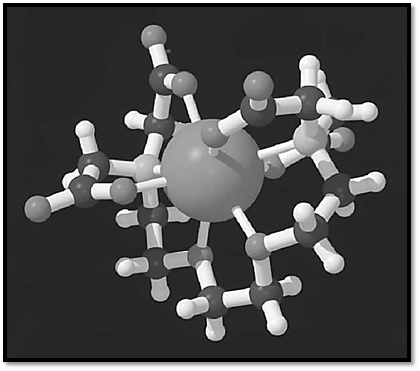
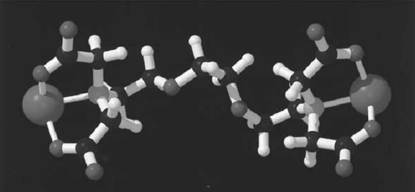
Figure 2. Three-dimensional structures of complexes of EGTA with (a) Ca2+ and (b) Mg2+.
EGTA as the free acid is a white crystalline powder and has a molecular weight of 340.35 Da. The affinities of EDTA and EGTA for several cations are compared in Table 1(6).
Table 1. Comparison of Affinities of EDTA and EGTA for Various Cations a
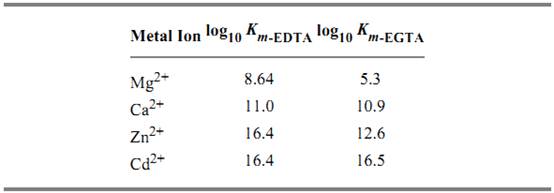
a The logarithms of their association constants (KM in units of M–1) are compared at pH 7.0, 25°C, and 0.1 M ionic strength (6)
References
1. A. Fabiato and F. Fabiato (1979) J. Physiol. (Paris) 75, 463.
2. A. Fabiato (1991) In Cellular Calcium: A Practical Approach, J. G. McCormack and P. H. Cobbold, eds., IRL Press, Oxford, pp. 159–176.
3. M. Otto, P. M. May, K. Murray, and J. D. Thomas (1985) Anal. Chem. 57, 1511–1517.
4. C. K. Schauer and O. P. Anderson (1987) J. Am. Chem. Soc. 109, 3646–3656.
5. C. K. Schauer and O. P. Anderson (1988) Inorg. Chem. 27, 3118–3130.
6. A. E. Martell and R. M. Smith (1974) Critical Stability Constants, Vol. 1, Plenum Press, New York.



|
|
|
|
تفوقت في الاختبار على الجميع.. فاكهة "خارقة" في عالم التغذية
|
|
|
|
|
|
|
أمين عام أوبك: النفط الخام والغاز الطبيعي "هبة من الله"
|
|
|
|
|
|
|
قسم شؤون المعارف ينظم دورة عن آليات عمل الفهارس الفنية للموسوعات والكتب لملاكاته
|
|
|