

النبات

مواضيع عامة في علم النبات

الجذور - السيقان - الأوراق

النباتات الوعائية واللاوعائية

البذور (مغطاة البذور - عاريات البذور)

الطحالب

النباتات الطبية


الحيوان

مواضيع عامة في علم الحيوان

علم التشريح

التنوع الإحيائي

البايلوجيا الخلوية


الأحياء المجهرية

البكتيريا

الفطريات

الطفيليات

الفايروسات


علم الأمراض

الاورام

الامراض الوراثية

الامراض المناعية

الامراض المدارية

اضطرابات الدورة الدموية

مواضيع عامة في علم الامراض

الحشرات


التقانة الإحيائية

مواضيع عامة في التقانة الإحيائية


التقنية الحيوية المكروبية

التقنية الحيوية والميكروبات

الفعاليات الحيوية

وراثة الاحياء المجهرية

تصنيف الاحياء المجهرية

الاحياء المجهرية في الطبيعة

أيض الاجهاد

التقنية الحيوية والبيئة

التقنية الحيوية والطب

التقنية الحيوية والزراعة

التقنية الحيوية والصناعة

التقنية الحيوية والطاقة

البحار والطحالب الصغيرة

عزل البروتين

هندسة الجينات


التقنية الحياتية النانوية

مفاهيم التقنية الحيوية النانوية

التراكيب النانوية والمجاهر المستخدمة في رؤيتها

تصنيع وتخليق المواد النانوية

تطبيقات التقنية النانوية والحيوية النانوية

الرقائق والمتحسسات الحيوية

المصفوفات المجهرية وحاسوب الدنا

اللقاحات

البيئة والتلوث


علم الأجنة

اعضاء التكاثر وتشكل الاعراس

الاخصاب

التشطر

العصيبة وتشكل الجسيدات

تشكل اللواحق الجنينية

تكون المعيدة وظهور الطبقات الجنينية

مقدمة لعلم الاجنة


الأحياء الجزيئي

مواضيع عامة في الاحياء الجزيئي


علم وظائف الأعضاء


الغدد

مواضيع عامة في الغدد

الغدد الصم و هرموناتها

الجسم تحت السريري

الغدة النخامية

الغدة الكظرية

الغدة التناسلية

الغدة الدرقية والجار الدرقية

الغدة البنكرياسية

الغدة الصنوبرية

مواضيع عامة في علم وظائف الاعضاء

الخلية الحيوانية

الجهاز العصبي

أعضاء الحس

الجهاز العضلي

السوائل الجسمية

الجهاز الدوري والليمف

الجهاز التنفسي

الجهاز الهضمي

الجهاز البولي


المضادات الميكروبية

مواضيع عامة في المضادات الميكروبية

مضادات البكتيريا

مضادات الفطريات

مضادات الطفيليات

مضادات الفايروسات

علم الخلية

الوراثة

الأحياء العامة

المناعة

التحليلات المرضية

الكيمياء الحيوية

مواضيع متنوعة أخرى

الانزيمات
Plate count method APHA 2001 for aerobic mesophilic bacteria in foods and water
المؤلف:
SILVA, N.D .; TANIWAKI, M.H. ; JUNQUEIRA, V.C.A.; SILVEIRA, N.F.A. , NASCIMENTO , M.D.D. and GOMES ,R.A.R
المصدر:
MICROBIOLOGICAL EXAMINATION METHODS OF FOOD AND WATE A Laboratory Manual
الجزء والصفحة:
21-3-2016
10765
Plate count method APHA 2001 for aerobic mesophilic bacteria in foods and water
The count can be performed using the pour plate, spread plate or membrane filtration method
1 - Material required for analysis
Preparation of the sample and serial dilutions
• Diluent: 0.1% Peptone Water (PW) or Butterfield’s Phosphate Buffer
• Dilution tubes containing 9 ml 0.1% Peptone Water (PW) or Butterfield’s Phosphate Buffer
• Observation: in which either the type or volume of diluent vary as a function of the sample to be examined.
Inoculation by pour plate
• Sterile, empty 20 × 100 mm Petri dishes
• Plate Count Agar (PCA)
• Orange Serum Agar (OSA) (for fruit juices)
• R2A or NWRI Agar (preferred for water samples)
Inoculation by spread plate
• Plates containing Plate Count Agar (PCA)
• Plates containing Orange Serum Agar (OSA) (for fruit juices)
• Plates containing R2A or NWRI Agar (for water samples)
• Glass or plastic spreaders (Drigalski loop) immersed in 70% ethanol Inoculation by membrane filtration
• Plates containing Plate Count Agar (PCA)
• Plates containing R2A, NWRI or m-HPC Agar (for water samples)
• Membranes 47 mm in diameter, porosity of 0.45μm, white and squared
• A previously sterilized filtration set
• Vacuum pump
• Tweezers – immersed in ethanol – for transferring the filter membranes
• Sterile 100 ml or 200 ml measuring cylinders, for determining the volume of the sample Incubation
• Laboratory incubator set at 35 ± 1°C (for foods in general)
• Laboratory incubator set at 30 ± 1°C (for fruit juices)
• Laboratory incubator set at 32 ± 1°C (for milk and dairy products)
2- Procedure
A general flowchart for enumeration of aerobic mesophilic bacteria in foods using the aerobic plate count method APHA 2001 is shown in Figure 6.1.
2.1 Pour plate technique
a) Preparation of the samples and serial dilutions..
b) Inoculation. Select three adequate dilutions of the sample and inoculate 1 ml of each dilution in separate, sterile and empty Petri dishes.
c) Addition of the culture medium. Pour 12–15 ml of previously melted and cooled to 44–46°C Plate Count Agar (PCA) onto the inoculated plates. In the case of fruit juices, replace PCA by Orange Serum Agar (OSA). For water samples, PCA should preferably be replaced by R2A or NWRI Agar. Mix the inoculum with the culture medium, in move-ments forming the number eight or in circular movements, eight to ten times clockwise and then eight to ten times counter-clockwise. Distribute the plates over the flat surface of a laboratory bench, without stacking, for the medium to solidify. For samples in which the occurrence of large and widely scattered colonies is common and expected (milk powder, for example) wait until the agar is completely solidified and cover with an overlay of the same medium.
d) Incubation. Invert the plates and incubate under the following conditions:
PCA – 35 ± 1°C/48 ± 2h for foods in general
PCA – 32 ± 1°C/48 ± 2h for dairy products
PCA – 32 ± 1°C/72 ± 3h for dried dairy products
OSA – 30 ± 1°C/48 ± 2h for fruit juices
R2A e NWRI – 35 ± 0.5°C/48 ± 2h for water
e) Counting the colonies and calculating the results. Select the plates with 25 to 250 colonies and count the colonies with the aid of a magnifying glass on a colony counter. Calculate the number of colony forming units (CFU) per gram or milliliter of the sample by multiplying the number of colonies by
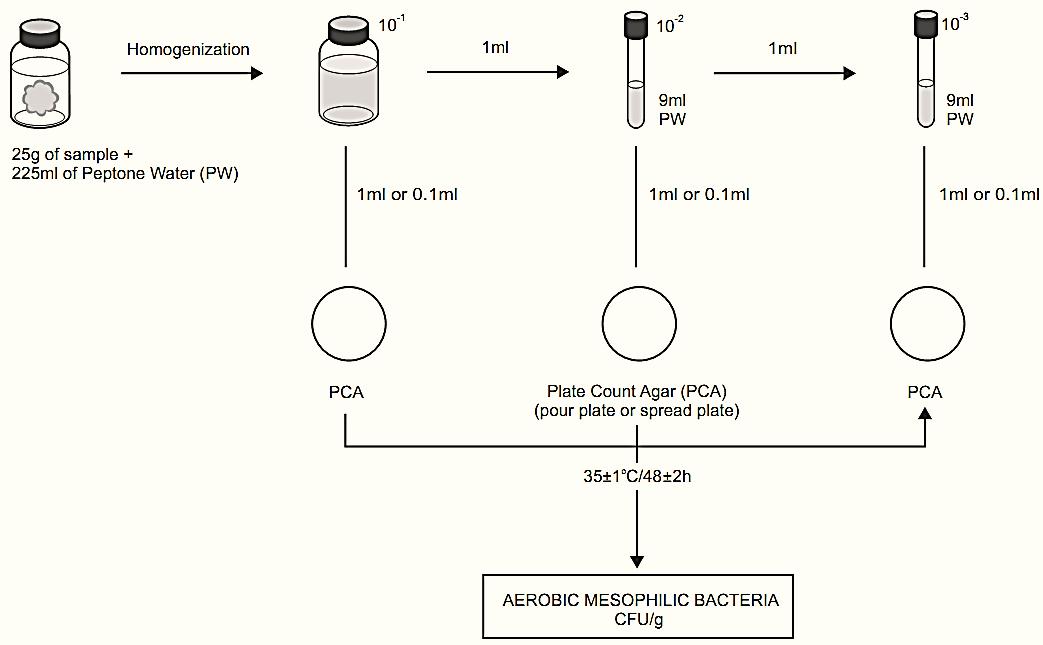
Figure 1 Scheme of analysis for enumeration of aerobic mesophilic bacteria in foods using the aerobic plate count method APHA 2001 (Morton, 2001).
the inverse of the inoculated dilution. If two plates were inoculated per dilution (duplicate), consider as the number of colonies the arithmetical average of the counts obtained in each of the plates of the duplicate. For counting colonies and calculating the results in unusual situations, refer to the guide-lines Detection limit of the standard procedure (1 ml/dilution): 1 CFU/ml for liquid samples or 10 CFU/g for solid samples.
2.2 Spread plate technique
a) Preparation of the samples and serial dilutions.
b) Preparation of the plates. Previously prepare plates containing Plate Count Agar (PCA) and, before use, dry. For samples of fruit juices, replace PCA by Orange Serum Agar (OSA). For water samples, PCA should preferably be replaced by R2A or NWRI Agar.
c) Inoculation. Select three adequate dilutions of the sample and inoculate 0.1 ml of each dilution onto the surface of the previously prepared plates. Spread the inoculum over the entire surface of the medium using a glass or plastic spreader (Drigalski), and continue until all excess liquid is absorbed. If the expected level of contamination is low, inoculate a greater volume (1 ml) of the first dilution, dividing this volume over four plates (inoculate three plates with 0.3 ml and one plate with 0.1 ml).
d) Incubation. Wait for the plates to dry, invert and incubate under the following conditions:
PCA – 35 ± 1°C/48 ± 2h for foods in general
PCA – 32 ± 1°C/48 ± 2h for dairy products
PCA – 32 ± 1°C/72 ± 3h for dried dairy products
OSA – 30 ± 1°C/48 ± 2h for fruit juices
R2A e NWRI – 35 ± 0.5°C/48 ± 2h for water
e) Counting the colonies and calculating the results. Follow the same guidelines described for pour plate but multiply the result by ten to account for the ten times smaller inoculation volume. If 1 ml of the first dilution is distributed over four plates, then the number of colonies of this dilution is the sum of the four plates. If the result is calculated based on the count of this dilution, then it is not necessary to multiply by ten.
Detection limit of the standard procedure (0.1 ml/dilution): 10 CFU/ml for liquid samples or 100 CFU/g for solid samples.
2.3 Membrane filtration technique
The membrane filtration method is mainly used for the examination of limpid or clear liquids, without solids in suspension. Membrane filtration is recommended for examining samples containing counts below the detection limit of other methods, and is frequently used to analyze carbonated soft drinks (which do not contain natural juices) and water intended for human consumption. It can also be used to examine samples of solid products converted into limpid, clear solutions such as salt and sugar.
a) Preparation of the filtration set.
b) Preparation of the plates. Previously prepare plates in the same manner as described for spread plate. For water samples, PCA should preferably be replaced by R2A, NWRI or m-HPC Agar. It is possible to perform the membrane filtration technique using 50 mm or 100 mm plates (containing 5 ml or 15–20 ml of culture medium, respectively) or sterile absorbent pads placed inside the (equally sterile) plates and soaked with 2 ml portions of the same media, in the liquid form.
c) Preparation of the samples. Measure 100 ml in a sterile measuring cylinder and carefully pour the content into the cup of the filtration set, avoiding spattering. If the cup of the filtration set is graduated and the graduation scale has a marking exactly matching the required volume, then the volume of the sample may be measured directly without using the measuring cylinder.
Note c.1) Since the filtration method is a technique that concentrates the microorganisms in samples with low counts, the usual procedure calls for filtering a total amount of 100 ml of the sample, which may be divided into two portions of 50 ml, four portions of 25 ml or three portions of 70, 25 and 5 ml, respectively. Selecting the volume to be filtered, however, will depend on the estimated level of contamination of the sample, so as to obtain plates containing a number of colonies within the 20 to 200 range.
Note c.2) When the volume to be filtered is smaller than 20 ml, add about 20–30 ml of diluent to the cup of the filtration set, before adding the sample. Accurate measuring of the volume of the diluent is not necessary, since its function is limited to merely increasing the volume to be filtered and facilitate obtaining a more even distribution of the microorganism on the membrane.
d) Filtration. Turn on the vacuum pump and start the filtration process. After passing the sample through the filtration membrane, and with the vacuum pump still running, rinse off the sides of the cup with 20 to 30 ml of the diluent, to collect contaminants that may have adhered to the surface. Repeat this procedure one more time. Turn off the vacuum pump before the membrane begins to dry excessively.
e) Transferring and incubating the membrane.
Remove the cup and, with a pair of flame-sterilized and subsequently cooled tweezers, transfer the membrane to the plate containing the culture medium, with the graph side of the membrane facing up. When placing the membrane onto the plate it is important that its entire surface becomes completely adhered to the medium, to ensure that the microorganisms come into contact with the nutrients contained in the medium. In case of bubble formation, the edge of the membrane that is closest to the bubble(s) should be gently lifted up and replaced in a way so as to eliminate the bubble(s).
f ) Incubation. Incubate the plates under the conditions described below in an inverted position and, preferably, placed inside bags or trays covered with moistened paper towels or filter paper, to avoid dehydration.
PCA – 35 ± 1°C/48 ± 2h for foods in general
R2A, NWRI or m-HPC – 35 ± 0.5°C/48 ± 2h for water
g) Counting the colonies and calculating the results. Begin counting the colonies with the aid of a stereoscopic microscope at a magnification of 10 to 15 times and, to facilitate visualization, place the plates into a position so as to obtain illumination perpendicular to the plane of the membrane. Select for the counting procedure plates containing 20 to 200 colonies. Follow the guide-lines below to count and calculate the number of CFU/ml:
If the number of colonies per square of the membrane is smaller than or equal to two, count all the colonies present and divide by the filtered volume to obtain the number of CFU/ml.
If the number of colonies per square of the membrane is in the range of three to ten, count ten squares and take the average per square. Multiply the average value by 100 and divide by the volume filtered to obtain the number of CFU/ml. If the number of colonies per square falls within the ten to twenty range, count only five small squares to take the average and calculate the number of CFU/ml in the same way.
If the number of colonies per square is greater than twenty, express the result as greater than 2.0 × 103 divided by the filtered volume. Detection limit of the standard procedure (filtration of 100 ml): 1 CFU/100 ml.
References
Silva, N.D .; Taniwaki, M.H. ; Junqueira, V.C.A.; Silveira, N.F.A. , Nasdcimento , M.D.D. and Gomes ,R.A.R .(2013) . Microbiological examination methods of food and water a laboratory Manual. Institute of Food Technology – ITAL, Campinas, SP, Brazil .
Morton, R.D. (2001) Aerobic plate count. In: Downes, F.P. & Ito, K. (eds). Compendium of Methods for the Microbiological Exami-nation of Foods. 4th edition. Washington, American Public Health Association. Chapter 6, pp. 63–67.
 الاكثر قراءة في البكتيريا
الاكثر قراءة في البكتيريا
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة
قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)


















