


 النبات
النبات
 الحيوان
الحيوان
 الأحياء المجهرية
الأحياء المجهرية
 علم الأمراض
علم الأمراض
 التقانة الإحيائية
التقانة الإحيائية
 التقنية الحيوية المكروبية
التقنية الحيوية المكروبية
 التقنية الحياتية النانوية
التقنية الحياتية النانوية
 علم الأجنة
علم الأجنة
 الأحياء الجزيئي
الأحياء الجزيئي
 علم وظائف الأعضاء
علم وظائف الأعضاء
 الغدد
الغدد
 المضادات الحيوية
المضادات الحيوية|
Read More
Date: 17-3-2016
Date: 6-3-2016
Date: 10-3-2016
|
Aerobic plate count
1.1 The importance and significance of the total aerobic mesophilic count
The Total Aerobic Mesophilic Plate Count, usually called Aerobic Plate Count or Standard Plate Count, is the most commonly used general indicator of bacterial populations in foods. The method does not differentiate types of bacteria, and is only used to obtain general information on the sanitary quality of products, manufacturing practices, raw materials, processing conditions, handling practices and shelf life.
The method is not an indicator of food safety, since it is not directly related to the presence of pathogens or toxins. Depending on the situation, the test can be useful for quality assessment purposes, since high bacterial populations may be indicative of sanitation deficiencies, flaws in process control systems, or contaminated ingredients. Fermented products, on the other hand, naturally contain high mesophilic populations, without any relation as to quality. The use of the total aerobic mesophilic plate count as a quality indicator should be carried out carefully. For example, when applied to ingredients, the test should be performed taking into account the dilution and its effect on the final product. When applied to dried foods, the total aerobic mesophilic plate count may indicate whether moisture is correctly managed and controlled during the drying process. Table.1 presents typical counts in some commonly internationally traded products. Table.2 depicts the FAO/WHO (Food and Agriculture Organization/World Health Organization) specifications for the total aerobic mesophilic plate counts in some foods. Table.3 shows the U.S. standards for the total aerobic mesophilic plate counts in milk and dairy products.
Table.1 Typical commodity mesophilic aerobic plate counts (Morton, 2001).

Table.2 FAO/WHO microbiological specifications for foods (Morton, 2001).
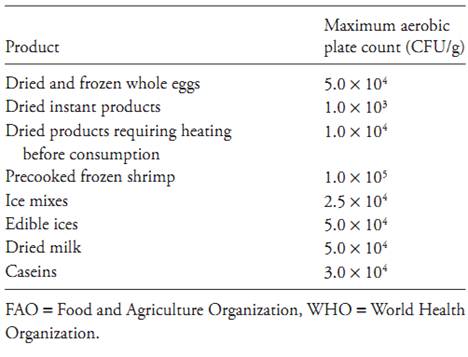
Table.3 U.S. standards for mesophilic aerobic plate count in milk and dairy products (Lewis et al., 2004).

1.2 Definition of psychrotrophics
Microorganisms that grow in foods under refrigeration (0–7°C) but have optimum growth at a temperature above 20°C are called psychrotrophics, psychrotrophs or psychrotrophiles. They are defined as microorganisms that are capable of producing visible growth at 7 + 1°C within a time span of seven to 10 days, irrespective of their optimum growth temperature. In the traditional systems which classify microorganisms as a function of temperature – thermophiles, mesophiles and psychrophiles – psychrotrophics are a subgroup of mesophiles, and not of psychrophiles, since the latter normally die at room temperature. Psychrotrophics, on the contrary, multiply in refrigerated foods, although they grow better within the mesophile temperature range.
The main psychrotrophic bacteria are distributed over several genera, including cocci and rods, spore forming and non-spore forming, aerobics and anaerobic. The most common in foods (dairy products, meat and meat products, poultry, fish and seafood) are species belonging to the following genera: Acinetobacter, Aeromonas, Alcaligenes, Arthrobacter, Bacillus, Brochothrix, Carnobacterium, Chromobacterium, Citrobacter, Clostridium, Corynebacterium, Enterobacter, Escherichia, Flavobacterium, Klebsiella, Lactobacillus, Leuconostoc, Listeria, Microbacterium, Micrococcus, Moraxella, Pseudomonas, Psychrobacter, Serratia, Shewanella, Streptococcus and Weissella. Alteromonas, Photobacterium and Vibrio are important fish spoilage bacteria. Species of Bacillus, Clostridium, Enterobacter, Flavobacterium, Pseudomonas and Yersinia cause softening and deterioration of refrigerated vegetables. Brochothrix, Lactobacillus, Leuconostoc along with members of the Enterobacteriaceae family cause spoilage of vacuum-packaged or modified atmosphere packaged foods, as do Carnobacterium and Weissella viridescens, but to a lesser extent.
Pseudomonas, Flavobacterium, Alcaligenes, Acinetobacter, Klebsiella, Bacillus and Lactobacillus cause the spoilage of dairy products, with Pseudomonas being the most encountered spoilage agent. Some pathogenic bacteria are also psychrotrophics, including Listeria monocytogenes, Yersinia enterocolitica, Aeromonas hydrophila, Vibrio cholerae, some strains of enteropathogenic E. coli, some strains of Bacillus cereus, and some non-proteolytic strains of Clostridium botulinum types E, B and F.
1.3 Methods of analysis
The classical total aerobic mesophilic or psychrotrophic count in foods Table.4 Analytical kits adopted as AOAC Official Methods for mesophilic aerobic plate count in foods

Compendium, is the standard plate count ( pour plate, spread plate or membrane filtration). The culture medium recommended for most tests is the Plate Count Agar (PCA), incubated at 35 ± 1°C/48 ± 2h, with the following exceptions:
For the analysis of milk and dairy products, recommends incubating PCA at 32 ± 1°C/48 ± 2h, extending incubation up to 72 ± 3h in the case of dried dairy products.
For the analysis of fruit juices, (Hatcher et al., 2001) recommends replacing PCA by Orange Serum Agar (OSA), incubated at 30 ± 1°C/48 ± 2h. OSA is a nutritionally richer medium than PCA, allowing the recovery of lactic bacteria normally present in these products. For the analysis of water, Section 9215 of the Standard Methods for the Examination of Water and Wastewater (Hunt and Rice, 2005) recommends replacing PCA by R2A agar or NWRI agar, incubated at 35 ± 0.5°C/48 ± 2h. These growth media may be used in pour plate, spread plate and membrane filtration. The same source further recommends, exclusively for the membrane filtration technique, the use of m-HPC agar, incubated at 35 ± 0.5°C/48 ± 2h. In water, the use of PCA as growth medium results in lower counts than when R2A or NWRI agar are used, although its use was maintained in the 21st edition of the Standard Methods for comparative studies with other media and for laboratories that need to ensure continuity and enable comparison with older records. Other methods that have already been officially recognized by the AOAC International are the microbiological test kits described in Table 4.
References
Silva, N.D .; Taniwaki, M.H. ; Junqueira, V.C.A.; Silveira, N.F.A. , Nasdcimento , M.D.D. and Gomes ,R.A.R .(2013) . Microbiological examination methods of food and water a laboratory Manual. Institute of Food Technology – ITAL, Campinas, SP, Brazil .
Morton, R.D. (2001) Aerobic plate count. In: Downes, F.P. & Ito, K. (eds). Compendium of Methods for the Microbiological Exami-nation of Foods. 4th edition. Washington, American Public Health Association. Chapter 6, pp. 63–67.
Hunt, M.E. & Rice, E.W. (2005) Microbiological examination. In: Eaton, A.D., Clesceri, L.S., Rice, E.W. & Greenberg, A.E. (eds). Standard Methods for the Examination of Water & Wastewater. 21st edition. Washington, American Public Health Association (APHA), American Water Works Association (AWWA) & Water Environment Federation (WEF). Part 9000, Section 9215, pp. 9.34–9.36.
Hatcher, W.S., Parish, M.E., Weihe, J.L., Splittstoesser, D.F. & Woodward, B.B. (2001) Fruit beverages. In: Downes, F.P. & Ito, K. (eds). Compendium of Methods for the Microbiological Exami-nation of Foods. 4th edition. Washington, American Public Health Association. Chapter 58, pp. 565–568.



|
|
|
|
دراسة يابانية لتقليل مخاطر أمراض المواليد منخفضي الوزن
|
|
|
|
|
|
|
اكتشاف أكبر مرجان في العالم قبالة سواحل جزر سليمان
|
|
|
|
|
|
|
المجمع العلمي ينظّم ندوة حوارية حول مفهوم العولمة الرقمية في بابل
|
|
|