


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 4-7-2017
Date: 5-5-2019
Date: 27-4-2019
|
Ionization energies and electron affinities
Ionization energies
The ionization energy of hydrogen
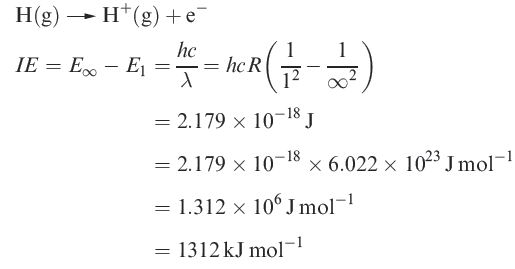
H atom has only one electron, no additional ionization processes can occur. For multi-electron atoms, successive ionizations are possible.
The first ionization energy, IE1, of an atom is the internal energy change at 0 K, Δ U(0 K), associated with the removal of the first valence electron (equation 1.17); the energy change is defined for a gas phase process. The units are kJ mol-1 or electron volts (eV).

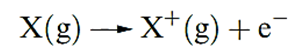 ( 1.1 )
( 1.1 )
It is often necessary to incorporate ionization energies into thermochemical calculations (e.g. Born–Haber or Hess cycles) and it is convenient to define an associated enthalpy change, ΔH(298K).
Since the difference between ΔH(298K) and ΔU(0K) is very small , values of IE can be used in thermochemical cycles so long as extremely accurate answers are not required.

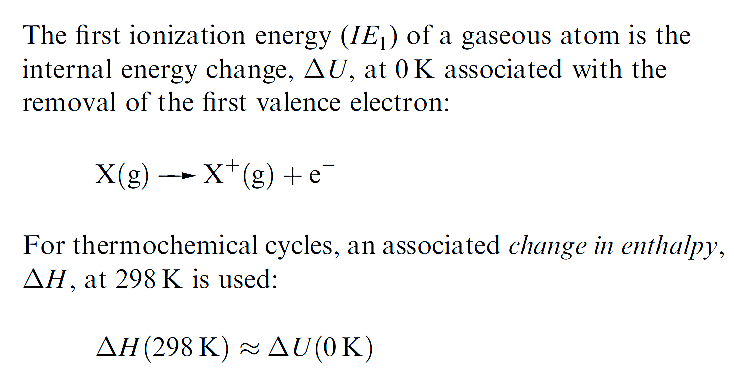
The second ionization energy, IE2, of an atom refers to step 1.2; note that this is equivalent to the first ionization of the ion X+. Equation 1.2 describes the step corresponding to the third ionization energy, IE3, of X, and successive ionizations are similarly defined:

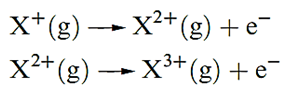 (1.2)
(1.2)
Figure 1. shows the variation in the values of IE1 as a function of Z. Several repeating patterns are apparent and some features to note are:
Each of these trends can be rationalized in terms of ground state electronic configurations. The noble gases (except for He) possess ns2 np6 configurations which are particularly stable and removal of an electron requires a great deal of energy. The ionization of a group 1 element involves loss of an electron from a singly occupied ns orbital with the resultant X ion possessing a noble gas configuration.
The general increase in IE1 across a given period is a consequence of an increase in Zeff. A group 15 element has a ground state electronic configuration ns2np3 and the np level is half-occupied. A certain stability is associated with such configurations and it is more difficult to ionize a group 15 element than its group 16 neighbour. In going from Be (group 2) to B (group 13), there is a marked decrease in IE1 and this may be attributed to the relative stability of the filled shell 2s2 configuration compared with the 2s2 2p1 arrangement; similarly, in going from Zn (group 12) to Ga (group 13), we need to consider the difference between 4s2 3d10 and 4s2 3d10 4p1 configurations.
The noble gases (except for He) possess ns2 np6 configurations which are particularly stable and removal of an electron requires a great deal of energy. The ionization of a group 1 element involves loss of an electron from a singly occupied ns orbital with the resultant X ion possessing a noble gas configuration.
The general increase in IE1 across a given period is a consequence of an increase in Zeff. A group 15 element has a ground state electronic configuration ns2 np3 and the np level is half-occupied. A certain stability is associated with such configurations and it is more difficult to ionize a group 15 element than its group 16 neighbour. In oing from Be (group 2) to B (group 13), there is a marked decrease in IE1 and this may be attributed to the relative stability of the filled shell 2s2 configuration compared with the 2s2 2p1 arrangement; similarly, in going from Zn (group 12) to Ga (group 13), we need to consider the difference between 4s2 3d10 and 4s2 3d10 4p1 configurations.
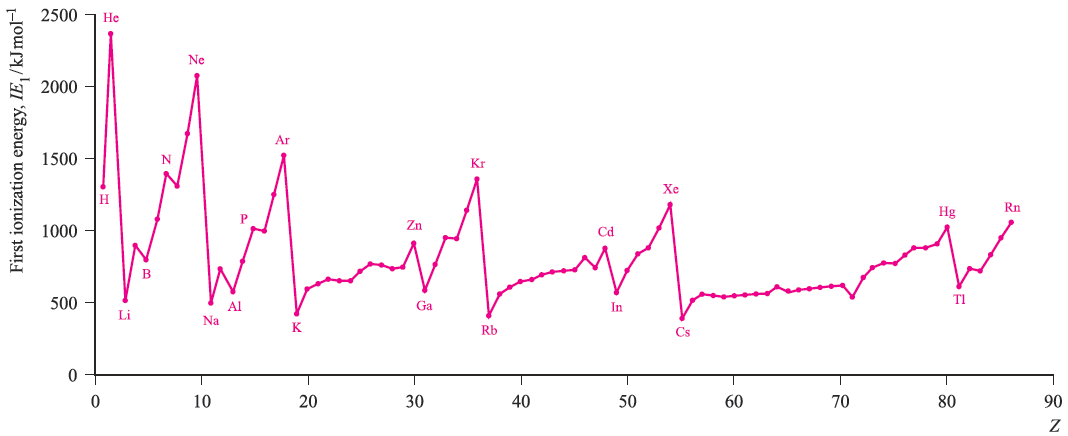
Fig. 1. The values of the first ionization energies of the elements up to Rn.



|
|
|
|
التوتر والسرطان.. علماء يحذرون من "صلة خطيرة"
|
|
|
|
|
|
|
مرآة السيارة: مدى دقة عكسها للصورة الصحيحة
|
|
|
|
|
|
|
نحو شراكة وطنية متكاملة.. الأمين العام للعتبة الحسينية يبحث مع وكيل وزارة الخارجية آفاق التعاون المؤسسي
|
|
|