


 النبات
النبات
 الحيوان
الحيوان
 الأحياء المجهرية
الأحياء المجهرية
 علم الأمراض
علم الأمراض
 التقانة الإحيائية
التقانة الإحيائية
 التقنية الحيوية المكروبية
التقنية الحيوية المكروبية
 التقنية الحياتية النانوية
التقنية الحياتية النانوية
 علم الأجنة
علم الأجنة
 الأحياء الجزيئي
الأحياء الجزيئي
 علم وظائف الأعضاء
علم وظائف الأعضاء
 الغدد
الغدد
 المضادات الحيوية
المضادات الحيوية|
Read More
Date: 4-4-2021
Date: 2-6-2021
Date: 10-12-2015
|
Cytochrome P450
Cytochromes P450 (designated here as P450) arise from a superfamily of genes that encode hemoproteins with molecular weights of 40 to 55 kDa and extraordinarily diverse properties and functions. P450 is nearly ubiquitous in the biotic world, with over 500 genes characterized in plants, animals, fungi, and bacteria, although it is not present in all organisms. Of the substrates of P450, 1000 are known, and there are perhaps as many as 106 in total; more than 40 different reactions are catalyzed (1). These enzymes play a critical role in:
1. Detoxification of bioactive molecules in vertebrates, insects, and plants
2. Metabolic activation of compounds that are both beneficial (drug precursors, hormones) and detrimental (carcinogens(
3. Biogenesis and catabolism of many endogenous compounds, including steroid hormones, vitamin D, biological insecticides, fatty acids, insect pheromones, and plant lignins and pigments
The P450 that metabolize xenobiotics appear to have no critical physiological functions, since mice with disrupted genes for these proteins had no obvious phenotypic abnormalities (2, 3). Most P450 proteins are membrane-bound in the endoplasmic reticulum (microsomes) or mitochondria, but soluble forms are present in bacteria. In mammals, the greatest concentrations of P450, particularly the detoxification forms, are present in the liver, but they are probably present in most tissues of the body, with relatively high concentrations in the intestines, lungs, nasal epithelia, and steroidogenic tissues. The genes for these enzymes differ in size, intron number, and chromosomal location. Gene expression of subsets of the P450 is regulated by a variety of substances, including xenobiotics such as aromatic hydrocarbons, barbiturates, and peroxisomal proliferators, and by endogenous steroid and polypeptide hormones.
The name cytochrome P450 is based on the characteristic increase in the absorbance of the protein heme group at 450 nm upon binding of CO and reduction of its iron atom. P450 differ structurally from most cytochromes because the fifth coordination group of the iron atom of the heme is occupied by a thiolate group from a cysteine residue and the sixth is occupied by water. They differ functionally because P450 are monooxygenases and not simply electron carriers.
1. Classification
On the basis of amino acid sequence similarity, the P450 are divided into more than 100 families and subfamilies (4). The various P450 within a gene family generally have >40% sequence identity, and members within a subfamily have identities >55% within mammalian species and >46% overall. P450 with >97% identity are arbitrarily considered allelic variants unless there is independent evidence for separate gene loci. Genes from animals, fungi, plants, or bacteria are assigned family numbers 1–49, 51–69, 71–99, and 101 and above, respectively. Family 51 is represented in plants and mammals, as well as fungi.
The recommended nomenclature for P450 genes is an italicized CYP (Cyp for mouse and Drosophila) followed by an Arabic number designating the family, a letter for the subfamily, and a second number for the member of the subfamily, eg, CYP1A1 (4). Identical terminology is used for gene products, except that the name is not italicized. Trivial names of P450 and other designations of the proteins, such as cytochrome P450 1A1, P450 1A1 or 1A1, are also considered acceptable in publications so long as the official name is specified.
2. Catalytic Activity
The overall reaction catalyzed by P450 is
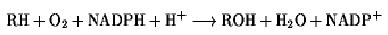
where R is an organic substrate. One oxygen atom of the O2 is incorporated into the substrate and the other is reduced to water, so P450 is both a monooxygenase and mixed-function oxidase. Further reactions and rearrangements lead to more than 40 different types of chemical reactions, including aliphatic and aromatic oxidations, N-, S-, and O-dealkylations, oxidative deamination, and peroxidation.
The P450 reaction cycle starts with the binding of the substrate to the ferric form of P450, which is followed by reduction of the heme iron atom by acceptance of an electron, binding of O2, activation of the oxygen by acceptance of a second electron, and scission of the oxygen molecule, followed by formation of water, a hydroxylated product, and ferric P450. The two electrons from NADPH are donated either to microsomal P450, through a flavoprotein, P450 reductase, or to mitochondrial and bacterial P450, sequentially through a flavoprotein (a ferredoxin reductase) and an iron-sulfur ferredoxin. In rare cases, the P450 and P450 reductase are fused into a single protein (5). Cytochrome b5 increases the activity of some P450 in vitro, either as a source of the second electron or allosterically (6).
3. Structure
The catalytic domain of membrane-bound P450 is oriented toward the cytoplasm for the microsomal forms and toward the matrix for the mitochondrial forms. The microsomal P450 are inserted into the membrane in a cotranslational, signal recognition particle-dependent manner (7). The N-terminal 20 to 25 amino acid residues function as a membrane-insertion and halt-transfer signal and are not cleaved, so they anchor the protein to the membrane. The mitochondrial P450 contain standard cleavable mitochondrial targeting signals at their N-terminus, but the mode of insertion into the inner membrane of the mitochondria is not known.
The three-dimensional structures of soluble bacterial P450, but not any membrane-bound P450, have been determined. Although the primary sequence is dissimilar among these bacterial proteins, a common “P450 fold” is present in each, which suggests that a similar core structure will also be present in eukaryotic membrane-bound P450 (8). The P450 structure contains an alpha-helical region and a beta-sheet region, which constitute about 70 and 20%, respectively, of the polypeptide chain. The sequence FXXGXXXCXG, which includes the heme-binding cysteine residue, is conserved in nearly all P450. Structures of eukaryotic P450 have been predicted from amino acid sequence alignments and homology molecular modeling with bacteria P450, and predictions of residues that interact with substrates have been generally consistent with the observed effects of mutations (9, 10).
4. Gene Structure
The sizes of the eukaryotic P450 genes, from the transcription initiation site to the polyadenylation site, vary dramatically, from about 3 kb for the CYP21 genes to >70 kb for CYP19 genes (11). In higher eukaryotes, the number of introns varies from 6 to 13, and the sites of insertion of introns are almost always conserved within a family, but only rarely between families. Polymorphism is common in P450 genes and underlies genetic differences in drug metabolism and congenital diseases in humans. The families of P450 genes are dispersed among at least nine chromosomes in humans and mice, but occasionally families cluster together, such as families 1, 11, and 19. Subfamilies within a family also may be dispersed to separate chromosomes or may cluster together, and members of the CYP2A, CYP2B, and CYP2F subfamilies are intermingled (4, 12). The gene duplication leading to the CYP21A locus included the C4 histocompatibility gene, so the two P450 genes alternate with the C4 gene. In spite of the diversity of P450 genes, their similarity in primary structures, the common three-dimensional fold, and some conserved intron sites among P450 genes support the idea that the P450 superfamily evolved by divergence from a common ancestral gene.
5. Gene Expression
P450 genes are expressed in tissue-, developmental-, and sex-specific patterns. The mechanisms regulating these patterns of expression are best understood in the liver and adrenal gland. An orphan receptor containing a zinc finger, termed Ad4BP or steroid factor-1 (SF-1), has been shown to be critical for the developmental and tissue-specific expression of P450 genes that are required for the biogenesis of adrenal and sex steroid hormones (13). The SF-1 gene has been disrupted in mice, with major effects on differentiation of the adrenal gland and gonads, pituitary gonadotropin function, and the ventral medial hypothalamus (14), so this gene has important developmental functions as well as effects on the expression of P450 genes. CYP19, aromatase, contains SF-1 sites, but its tissue-specific expression is also dependent on alternate promoters and transcription initiation sites (15).
All the known liver-enriched regulatory factors have been implicated in the expression of one or more P450 genes. Examples in which functional binding sites for liver-enriched factors have been demonstrated include HNF-1a for CYP2E1, HNF-4 for CYP2C1/2/3, DBP for CYP2C6 and CYP7, HNF-3 for CYP2C6, C/EBPb for CYP2D5, and C/EBPa and C/EBPb for CYP2B1/2 (16, 17). A variety of ubiquitously expressed factors also contribute to basal expression of the CYP genes. The developmental expression of CYP2C6 correlates with the developmental activation of DBP (16), and the diurnal expression of DBP underlies the diurnal regulation of CYP7 (18). Activation of CYP2D5 by C/EBPb also requires the constitutive factor Sp1, which has a binding site near that of C/EBPb (16) .
5.1. Sex-dependent Expression
The sex-dependent expression of several P450 genes in rodents may be mediated by more than one mechanism. In rats, the sex-specific expression of CYP genes is dependent on different patterns of growth hormone secretion in the two sexes. STAT transcription factors (19) and phospholipaseA2 (20) may be involved in the growth hormone-regulated male-specific expression of specific CYP genes, but additional studies are required to establish the exact mechanism. In mice, a sex difference information (SDI) element is present in male-specific Cpy2d9 and female-specific Cyp2a4 (21). The SDI contains a CpG sequence that is methylated in a sex-dependent way, and a factor has been identified that binds in a methylation-dependent manner (22). The exact role of this factor is not yet resolved, as its tissue and developmental expression differs from that of the CYP genes.
5.2. Aromatic Hydrocarbons
The expression of subsets of the xenobiotic-metabolizing P450 genes is regulated by aromatic hydrocarbons, peroxisomal proliferators, and barbiturates. The induction of CYP1A1 by aromatic hydrocarbons, including the halogenated aromatic hydrocarbon 2,3,7,8-tetrachlorodibenzo-p-dioxin, is best understood. Genetic differences in the induction of Cyp1a1 in mice and in variants of cultured mouse hepatoma Hepa 1 cells contributed critically to the identification of a regulatory locus AhR, which encodes the aromatic hydrocarbon receptor, and a second gene, the Ah receptor nuclear translocator Arnt, which encodes a protein required for the nuclear import of AhR (23, 24). The AhR is complexed with the molecular chaperone hsp90 in the cytoplasm in uninduced cells, which is essential to maintain a functional AhR. Binding of the ligand results in the dissociation of hsp90 and localization of AhR in the nucleus. Arnt is a nuclear protein that binds to liganded AhR and is required for the binding of AhR to the DNA binding site (25). AhR/Arnt bind to multiple sites within an enhancer about 1 kb from the start site of transcription and disrupt the nucleosomal structure of the enhancer (23). In turn, the nucleosomal structure of the promoter region is also disrupted in a manner dependent on the transactivation domain of AhR, which then allows the binding of regulatory proteins to the proximal promoter. The core sequence of the binding sites, which have been designated xenobiotic-, dioxin-, or Ah-response elements by different investigators, is 5′-TNGCGTG-3′. Arnt binds to the core sequence, and AhR binds to the flanking sequence. Both AhR and Arnt contain (1) basic loop-helix-loop (bLHL) motifs near their N-termini; (2) PAS domains, which exhibit homology with the Drosophila proteins, Per and Sim; and (3) glutamine-rich, putative transcriptional activation domains in the C-terminal region (24). The ligand and hsp90 interact with sequences near the PAS domain of AhR, and the binding of AhR to Arnt involves the bHLH and PAS domains of each protein. Arnt is capable of transactivation independently, but AhR transactivation requires the association of AhR with Arnt.
5.3. Peroxisomal Proliferators
Induction of expression of the CYP4A genes by peroxisomal proliferators and fatty acids requires peroxisomal proliferator-activated receptor-a (PPARa), a member of the steroid hormone receptor family (26). CYP4A P450 catalyze the w-hydroxylation of fatty acids, including arachidonic acid, which may ultimately result in the degradation of the fatty acids by peroxisomes. The induction of CYP4A genes is defective in mice with disrupted PPARa genes (27). PPARa forms a heterodimer with retinoid-X-receptor (RXR) to activate the gene. The predominant sequence in Cyp4A6 that confers the response to peroxisomal proliferators contains an imperfect repeat of the sequence AGGTCA, with a single base pair between the repeats and additional sequence to the 5′ side of the repeat. The 5′ sequence appears to be important for the specificity of binding of PPARa/RXRa to the nonconsensus, imperfect repeat of the peroxisomal proliferator response element present in CYP4A6 (26) .
5.4. Barbiturates
Induction of P450 genes by barbiturates is not well understood. Neither a barbiturate receptor nor specific barbiturate response elements in mammalian genes have been defined, and different mechanisms may be involved for different P450 (28). The system best understood is the induction by barbiturates of Bm-1, Bm-2, and Bm-3 P450 in Bacillus megaterium (29). In untreated cells, a repressor, Bm3R1, binds to a palindromic 20-bp sequence upstream of a promoter that drives the expression of both the repressor and Bm-3. In the presence of barbiturate, the binding is reversed, which may result from direct effects on the repressor, but positively acting factors are also induced that compete for the binding of the repressor at the palindromic site and at sites termed “barbie boxes” present in the 5′ regions of the Bm-3 and Bm-1 genes. In the mammalian CYP2B1/2 genes, evidence has been presented for phenobarbital responsive sequences in both the proximal promoter region, including barbie-box-like sequences (30), and in distal phenobarbital-responsive enhancers (31, 32) . Identification of specific responsive elements and characterization of the cognate binding proteins will be required to establish the role of these regions in phenobarbital induction.
5.5. Glucocorticoids
A number of CYP genes are also modulated by glucocorticoids. In some cases, the effect is mediated through the classical glucocorticoid receptors, such as modulation by glucocorticoids of aromatic hydrocarbon induction of CYP1A1 and phenobarbital induction of CYP2B genes (33). In other cases, such as CYP3A genes, the response to glucocorticoids occurs by nonclassical mechanisms that remain to be elucidated (34).
5.6. Cyclic AMP
Genes for P450 involved in steroid metabolism are induced by polypeptide hormones via cyclic AMP-mediated (cAMP) mechanisms (35). In the adrenal gland, CYP11A, CYP11B, CYP17, and CYP21 are regulated in this way. Interestingly, even though each of these genes probably evolved from a common ancestor, different regulatory elements in the promoter of each gene mediate the cAMP response. A classical CREB binding site is present in CYP11B, but binding sites for proteins
other than CREB are present in other genes, and different sites are present in orthologous genes in different species. In bovine CYP11A, a binding site for Sp1, a constitutive factor, mediates the cAMP response (36), and members of the homeodomain PBX family of helix-turn-helix motif proteins bind to the cAMP-responsive element in CYP17 (37). Multiple proteins bind to many of these elements, and those that predominate in vivo remain to be identified.
References
1. M. J. Coon, A. D. N. Vaz, and L. L. Bestervelt (1996) FASEB J. 10, 428–434.
2. H. C. L. Liang et al. (1996) Proc. Natl. Acad. Sci. USA 93, 1671–1676.
3. S. S. T. Lee, J. T. M. Buters, T. Pineau, P. Fernandez-Salguero, and F. J. Gonzalez (1996) J. Biol. Chem. 271, 12063–12067.
4. D. R. Nelson et al. (1996) Pharmacogenetics 6, 1–42.
5. L. O. Narhi and A. J. Fulco (1987) J. Biol. Chem. 262, 6683–6690.
6. H. Yamazaki, W. W. Johnson, Y.-F. Ueng, T. Shimada, and F. P. Guengerich (1996) J. Biol. Chem. 271, 27438–27445.
7. B. Kemper and E. Szczesna-Skorupa (1989) Drug Metabol. Rev. 20, 811–820.
8. C. A. Hasemann, R. G. Kurumbail, S. S. Boddupalli, J. A. Peterson, and J. Deisenhofer (1995( Structure 2, 41–62.
9. O. Gotoh (1992) J. Biol. Chem. 267, 83–90.
10. C. von Wachenfeldt and E. F. Johnson (1995) In Cytochrome P450—Structure, Mechanism, and Biochemistry (P. Ortiz de Montellano, ed.), Plenum Press, New York, pp. 183–223.
11. B. Kemper (1993) In Frontiers in Biotransformation (K. Ruckpaul and H. Rein, eds.), Vol. 8, Akkademie Verlag, Berlin, pp. 1–58.
12. S. M. G. Hoffman, P. Fernandez-Salguero, F. J. Gonzalez, and H. W. Mohrenweiser (1995) J. Mol. Evol. 41, 894–900.
13. D. S. Lala, D. A. Rice, and K. L. Parker (1992) Mol. Endocrinol. 6, 1249–1258.
14. K. L. Parker and B. P. Schimmer (1996) Trends Endo. Metab. 7, 203–207.
15. M. S. Mahendroo, C. R. Mendelson, and E. R. Simpson (1993) J. Biol. Chem. 268, 19463–19471.
16. F. J. Gonzalez and Y.-H. Lee (1996) FASEB J. 10, 1112–1118.
17. Y. Park and B. Kemper (1996) DNA Cell Biol. 15, 693–701.
18. D. J. Lavery and U. Schibler (1993) Genes Develop. 7, 1871–1884.
19. D. J. Waxman, P. A. Ram, S. H. Park, and H. K. Choi (1995) J. Biol. Chem. 270, 13262–13270.
20. P. Tollet, M. Hamberg, J. Å. Gustafsson, and A. Mode (1995) J. Biol. Chem. 270, 12569–12577.
21. H. Yoshioka, M. Lang, G. Wong, and M. Negishi (1990) J. Biol. Chem. 265, 14612–14617.
22. N. Yokomori, R. Kobayashi, R. Moore, T. Sueyoshi, and M. Negishi (1995) Mol. Cell Biol. 15, 5355–5362 .
23. J. P. Whitlock Jr. et al. (1996) FASEB J. 10, 809–818.
24. O. Hankinson (1995) Ann. Rev. Pharmacol. Toxicol. 35, 307–340.
25. R. S. Pollenz, C. A. Sattler, and A. Poland (1993) Mol. Pharmacol. 45, 428–438.
26. E. F. Johnson, C. N. A. Palmer, K. J. Griffin, and M.-H. Hsu (1996) FASEB J. 10, 1241–1249.
27. S. S.-T. Lee et al. (1995) Mol. Cell Biol. 15, 3012–3022.
28. D. J. Waxman and L. Azaroff (1992) Biochem. J. 281, 577–592.
29. Q. Liang and A. J. Fulco (1995) J. Biol. Chem. 270, 18606–18615.
30. L. Prabhu et al. (1995) Proc. Natl. Acad. Sci. USA 92, 9628–9632.
31. E. Trottier, A. Belzil, C. Stoltz, and A. Anderson (1995) Gene 158, 263–268.
32. Y. Park, H. Li, and B. Kemper (1996) J. Biol. Chem. 271, 23725–23728.
33. R. A. Prough, M. W. Linder, J. A. Pinaire, G.-H. Xiao, and K. C. Falkner (1996) FASEB J. 10, 1369-1377.
34. L. C. Quattrochi, A. S. Mills, J. L. Barwick, C. B. Yockey, and P. S. Guzelian (1995) J. Biol. Chem. 270, 28917–28924.
35. M. Waterman (1994) J. Biol. Chem. 269, 27783–27786.
36. P. Venepally and M. Waterman (1995) J. Biol. Chem. 270, 25402–25411.
37. N. Kagawa, A. Ogo, Y. Takahashi, A. Iwamatsu, and M. R. Waterman (1994) J. Biol. Chem. 269, 18716–18719.



|
|
|
|
تفوقت في الاختبار على الجميع.. فاكهة "خارقة" في عالم التغذية
|
|
|
|
|
|
|
أمين عام أوبك: النفط الخام والغاز الطبيعي "هبة من الله"
|
|
|
|
|
|
|
الأمين العام للعتبة العسكرية المقدسة يستقبل قائد الفرقة الرابعة الشرطة الاتحادية
|
|
|