


 النبات
النبات
 الحيوان
الحيوان
 الأحياء المجهرية
الأحياء المجهرية
 علم الأمراض
علم الأمراض
 التقانة الإحيائية
التقانة الإحيائية
 التقنية الحيوية المكروبية
التقنية الحيوية المكروبية
 التقنية الحياتية النانوية
التقنية الحياتية النانوية
 علم الأجنة
علم الأجنة
 الأحياء الجزيئي
الأحياء الجزيئي
 علم وظائف الأعضاء
علم وظائف الأعضاء
 الغدد
الغدد
 المضادات الحيوية
المضادات الحيوية|
Read More
Date: 15-10-2021
Date: 18-10-2021
Date: 28-11-2021
|
Bioenergetics and Oxidative Phosphorylation
The change in free energy (ΔG) occurring during a reaction predicts the direction in which that reaction will spontaneously proceed. If ΔG is negative (that is, the product has a lower free energy than the substrate), then the reaction is spontaneous as written. If ΔG is positive, then the reaction is not spontaneous. If ΔG = 0, then the reaction is in equilibrium.
The ΔG of the forward reaction is equal in magnitude but opposite in sign to that of the back reaction. The ΔG are additive in any sequence of consecutive reactions, as are the standard free energy changes (ΔG0). Therefore, reactions or processes that have a large, positive ΔG are made possible by coupling with those that have a large, negative ΔG such as ATP hydrolysis. The reduced coenzymes nicotinamide adenine dinucleotide (NADH) and flavin adenine dinucleotide (FADH2) each donate a pair of electrons to a specialized set of electron carriers, consisting of flavin mononucleotide (FMN), iron-sulfur centers, coenzyme Q, and a series of heme-containing cytochromes, collectively called the electron transport chain. This pathway is present in the inner mitochondrial membrane (impermeable to most substances) and is the final common pathway by which electrons derived from different fuels of the body flow to oxygen (O2), which has a large, positive reduction potential (E0), reducing it to water. The terminal cytochrome, cytochrome c oxidase, is the only cytochrome able to bind O2. Electron transport results in the pumping of protons (H+) across the inner mitochondrial membrane from the matrix to the intermembrane space, 10 H+ per NADH oxidized. This process creates electrical and pH gradients across the inner mitochondrial membrane. After H+ have been transferred to the cytosolic side of the membrane, they reenter the matrix by passing through the Fo H+ channel in ATP synthase (Complex V), dissipating the pH and electrical gradients and causing conformational changes in the F1 β subunits of the synthase that result in the synthesis of ATP from ADP + inorganic phosphate. Electron transport and phosphorylation are tightly coupled in oxidative phosphorylation ([OXPHOS] Fig. 1). Inhibition of one process inhibits the other. These processes can be uncoupled by uncoupling protein-1 of the inner mitochondrial membrane of brown adipocytes and by synthetic compounds such as 2,4-dinitrophenol and aspirin, all of which dissipate the H+ gradient. In uncoupled mitochondria, the energy produced by electron transport is released as heat rather than being used to synthesize ATP.
Mutations in mitochondrial DNA, which is maternally inherited, are responsible for some cases of mitochondrial diseases such as Leber hereditary optic neuropathy. The release of cytochrome c into the cytoplasm and subsequent activation of proteolytic caspases results in apoptotic cell death.
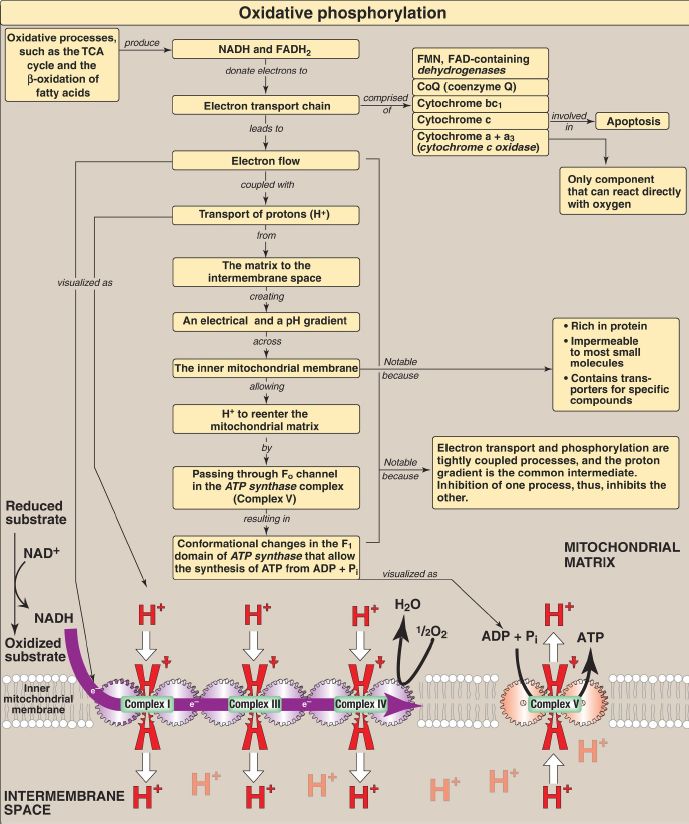
Figure 1: Key concept map for oxidative phosphorylation (OXPHOS). [Note: Electron (e−) flow and ATP synthesis are shown as sets of interlocking gears to emphasize coupling.] TCA = tricarboxylic acid; NAD(H) = nicotinamide adenine dinucleotide; FAD(H2) = flavin adenine dinucleotide; FMN = flavin mononucleotide; ADP = adenosine diphosphate.



|
|
|
|
لخفض ضغط الدم.. دراسة تحدد "تمارين مهمة"
|
|
|
|
|
|
|
طال انتظارها.. ميزة جديدة من "واتساب" تعزز الخصوصية
|
|
|
|
|
|
|
مشاتل الكفيل تزيّن مجمّع أبي الفضل العبّاس (عليه السلام) بالورد استعدادًا لحفل التخرج المركزي
|
|
|