


 الفيزياء الكلاسيكية
الفيزياء الكلاسيكية
 الكهربائية والمغناطيسية
الكهربائية والمغناطيسية
 علم البصريات
علم البصريات
 الفيزياء الحديثة
الفيزياء الحديثة
 النظرية النسبية
النظرية النسبية
 الفيزياء النووية
الفيزياء النووية
 فيزياء الحالة الصلبة
فيزياء الحالة الصلبة
 الليزر
الليزر
 علم الفلك
علم الفلك
 المجموعة الشمسية
المجموعة الشمسية
 الطاقة البديلة
الطاقة البديلة
 الفيزياء والعلوم الأخرى
الفيزياء والعلوم الأخرى
 مواضيع عامة في الفيزياء
مواضيع عامة في الفيزياء|
Read More
Date: 23-3-2021
Date: 25-3-2021
Date: 22-3-2021
|
HYDROGEN ATOM SPECTRUM
The emission or absorption spectrum of hydrogen gas and many other gases (He, Ne, Xe, etc.) was a subject of intense research for more than two decades at the beginning of the twentieth century. In fact, observation of such a spectrum in the scientific laboratories was the observation of the earliest “quantum effect.” Interestingly, no one had a clue about what caused such a spectrum, and scientists were puzzled over its existence. The explanation for its origin came later and laid the foundation of the preliminary version of the quantum mechanics. The learning of quantum mechanics will be incomplete without knowing the story behind the spectrum of hydrogen gas, so let us begin here with a short story on the origin of hydrogen atom spectrum….
It was George Claude in 1910, a French chemist and inventor, who invented the first electrical discharge tube, known today simply as neon light. An electrical discharge tube is a sealed glass tube with a metal electrode at each end, filled with gases such as neon, helium or xenon at low pressure. When voltage is applied between the electrodes, the tube glows with a color that depends on the type of gas contained in the tube. Hydrogen gas in the tube gives a pink color, neon gas gives an orange-to-red color, and helium gives white, gray or blue colors. What causes such a glow and different colors from different gases? The scientists at that time were struggling to explain this phenomenon of the electric discharge tube. It took a decade or so to fully understand it. Now, using quantum mechanics principles, this phenomenon can be easily explained, and it led to the understanding of the hydrogen atom spectrum and the spectrum of many other gases. We will apply the understanding of the structure of the hydrogen atom that we have learned so far for explaining this phenomenon.
As discussed in the previous sections, the energy levels of the hydrogen atom are discrete. According to quantum mechanics, these energy levels are given by the following equation:
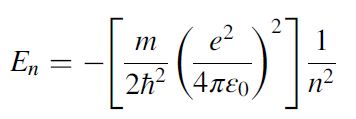
When an electron jumps from a higher-energy level to a lower-energy level, it emits a photon that carries energy that is equal to the energy of the difference between the two levels and is given as:
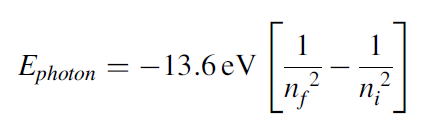
Going back to the discharge tube, when voltage is applied between the electrodes of the discharge tube, the H2 molecules of the gas dissociate into hydrogen atoms, and such atoms then collide with each other and with the walls of the tube, which causes electrons in the hydrogen atoms to jump from a higher-energy level to a lower-energy level, emitting radiation or photons that carry energy equivalent to the energy difference between the levels. The glow and color of the radiation observed in the tube is due to the most intense radiation emitted by these electrons. When the light coming through the discharge tube is made to pass through a prism, the spectrum of the light is observed. For hydrogen gas, there are four narrow discrete bands or lines, as shown in the figure below. Each line corresponds to the radiation emitted by electrons from a higher level to a lower level, as shown in the Figure 1. This line spectrum is very distinct from the spectrum of continuous bands of colors obtained from the light of a hot filament. This observation of the line spectrum instead of the continuous band spectrum of the light coming from discharge tube puzzled scientists at that time. The discreteness in the spectrum of the narrow bands mainly arises due to discreteness in the atomic structure of the atoms. The atomic structure of an atom is its signature characteristic, which is revealed in the line spectrum. Every atom is completely distinct from the other atoms due to its atomic structure. Thus, by looking at the line spectrum of the light, the information about the source can be revealed. This is precisely how the source of light coming from the stars was identified, and the first lines of hydrogen spectra were found not in laboratory but in the spectra of light from stars. In 1881, Sir William Huggins identified 10 lines of hydrogen emission spectra by looking at the first photographs of stellar spectra. These lines varied in wavelength from the red visible light to the near ultraviolet light. In 1885, working from astronomical measurements, Johann Jakob Balmer found that he could account for the positions of all known lines by applying a simple
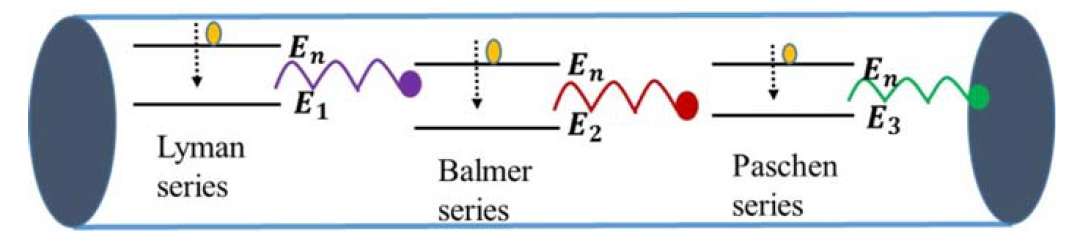
FIGURE 1: Electrical discharge tube or simply “neon light” showing several electronic transitions in hydrogen atoms taking place, causing observation of line spectrum.
empirical formula. The entire set of lines has come to be known as Balmer series. Another group of series in the far ultraviolet region are called Lyman series and Paschen series. The Balmer series arises due to transitions taking place from any higher level “n” to a lower level 2, as shown in the Figure 1. All lines in the series share the same lower state. Similarly, the Lyman and Paschen series also share the same lower states. These lines can be further split into closer lines that result mainly from relativistic and magnetic interactions related to orbital and spin angular momentum of the electrons of the atom. This splitting is called fine structure of the spectrum. There is so much that can be revealed about the hydrogen atom by studying it atomic spectrum. New findings about the hydrogen spectrum will continue as news tools are becoming available for obtaining spectrum. For further details about the spectrum of hydrogen, a reference is mentioned in references section of the book (Figures 1 and 2).

FIGURE 2: On the left hand side is a hydrogen spectral tube excited by a 5000-volt transformer. The three prominent lines observed from the spectra of the radiation obtained from the tube are shown at the right of the image through a 600 lines/mm diffraction grating. These are Balmer lines in the visible range of the spectrum.



|
|
|
|
دراسة يابانية لتقليل مخاطر أمراض المواليد منخفضي الوزن
|
|
|
|
|
|
|
اكتشاف أكبر مرجان في العالم قبالة سواحل جزر سليمان
|
|
|
|
|
|
|
المجمع العلمي ينظّم ندوة حوارية حول مفهوم العولمة الرقمية في بابل
|
|
|