


 النبات
النبات
 الحيوان
الحيوان
 الأحياء المجهرية
الأحياء المجهرية
 علم الأمراض
علم الأمراض
 التقانة الإحيائية
التقانة الإحيائية
 التقنية الحيوية المكروبية
التقنية الحيوية المكروبية
 التقنية الحياتية النانوية
التقنية الحياتية النانوية
 علم الأجنة
علم الأجنة
 الأحياء الجزيئي
الأحياء الجزيئي
 علم وظائف الأعضاء
علم وظائف الأعضاء
 الغدد
الغدد
 المضادات الحيوية
المضادات الحيوية|
Read More
Date: 18-1-2021
Date: 16-1-2021
Date: 21-12-2020
|
Amperometric Biosensors
Enzyme-catalysed redox reactions can form the basis of a major class of biosensors if the flux of redox electrons can be determined. Normally a constant potential is applied between two electrodes and the current, due to the electrode reaction, is determined. The potential is held relative to that of a reference electrode and is chosen such that small variations do not affect the rate of the electrode reaction. Direct electron transfer between the electrode and the redox site associated with the enzyme is kinetically hindered due to the distance between them or the presentation of an unfavourable pathway for the electrons. This can usually be addressed by the use of mediators and/or special electrode materials.
The first and simplest biosensor was based on this principle. It was for the determination of glucose and made use of the Clark oxygen electrode. Figure 1. shows a section through such a simple amperometric biosensor. A potential of -0.6V is applied between the central platinum cathode and the surrounding silver/silver chloride reference electrode (the anode). Dissolved molecular oxygen at the platinum cathode is reduced and the circuit is completed by means of the saturated KCl solution. Only oxygen can be reduced at the cathode due to its covering by a thin Teflon or polypropylene membrane through which the oxygen can diffuse but which acts as a barrier to other electroactive species.
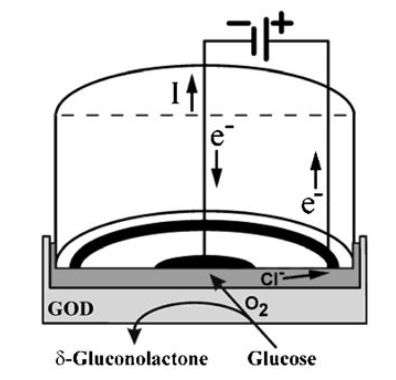
Figure 1. Amperometric glucose biosensor based on the oxygen electrode utilising glucose oxidase (GOD).
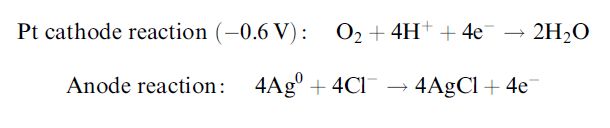
The biocatalyst is retained next to the electrode by means of a membrane, which is permeable only to low molecular weight molecules including the reactants and products.
Glucose may be determined by the reduction in the dissolved oxygen concentration when the redox reaction, catalysed by glucose oxidase, occurs (Figure 2.a):
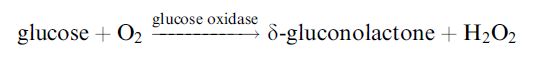
It is fortunate that this useful enzyme is also one of the most stable oxidoreductases found. Conditions can be chosen such that the rate at which oxygen is lost from the biocatalyst-containing compartment is proportional to the bulk glucose concentration. Other oxidases can be used in this biosensor configuration and may be immobilised as part of a membrane by treatment of the dissolved enzyme(s), together with a diluent protein, with glutaraldehyde on a cellulose or nylon support. An alternative method of determining the rate of reaction is to detect the hydrogen peroxide produced directly by reversing the polarity of the electrodes (Figure 2.b). This is the principle used in YSI (Yellow Springs) analysers.
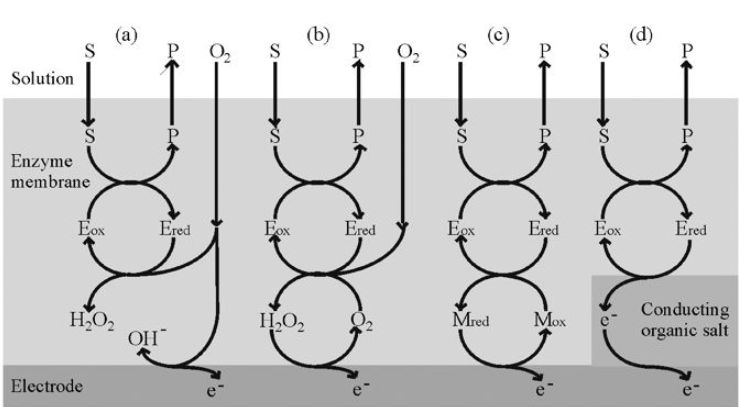
Figure 2. The redoxmechanisms for various amperometric biosensor configurations.
The use of a covering, such as the highly anionic Nafion membrane, prevents electroactive anions such as ascorbate reaching the electrode without restricting glucose. Internally, a cellulose acetate membrane replaces the Teflon membrane to allow passage of the
hydrogen peroxide. This arrangement has a higher sensitivity than that utilising an oxygen electrode but, in the absence of highly selective membranes, is more prone to interference at the electrode surface.

These electrodes can be developed further for the determination of substrates for which no direct oxidase enzyme exists. Thus sucrose can be determined by placing an invertase layer over the top of the glucose oxidase membrane in order to produce glucose (from the sucrose), which can then be determined. Interference from glucose in the sample can be minimised by including a thin anti-interference layer of glucose oxidase and peroxidase over the top of both layers, which removes the glucose without significantly reducing the oxygen diffusion to the electrode. A clever alternative approach to assay sucrose and glucose together makes use of the lag period in the response due to the necessary inversion of the sucrose that delays its response relative to the glucose (Figure 3). This arrangement shows the typical craft required for using existing biosensors to analyse alternative analytes.

Figure 3. A kinetically controlled anti-interference membrane for sucrose. The sample is presented as a rapid pulse of material in the flowing stream. Use of phosphate buffer, which catalyses mutarotation, removes the need for the mutarotase enzyme.
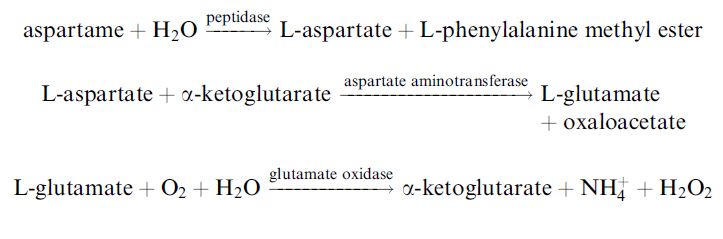
Many biochemicals may be analysed using similar biosensors. One interesting example is the biosensor for determining the artificial sweetener aspartame in soft drinks, where three enzymes are necessary in the sensing membrane in order to produce the H2O2 for the electrode
reaction:
Fish freshness can be determined using a similar concept; the nucleotides in fish changing due to a series of reactions after death. Fish freshness can be quantified in terms of its K value, where
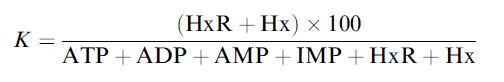
where HxR, IMP and Hx represent inosine, inosine-5´-monophosphate and hypoxanthine, respectively. After fish die, their ATP undergoes catabolic degradation through a series of reactions:
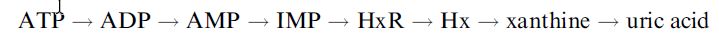
The accumulation of the intermediates inosine and hypoxanthine relative to the nucleotides is an indicator of how long the fish has been dead and its handling and storage conditions and hence its freshness. A commercialised fish freshness biosensor has been devised which utilises
a triacylcellulose membrane containing immobilised nucleoside phosphorylase and xanthine oxidase over an oxygen electrode:
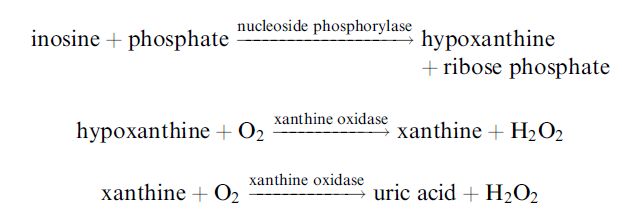
The electrode may be used to determine the reduction in oxygen or the increase in hydrogen peroxide. The inosine content may be determined after the hypoxanthine content by the addition of the necessary phosphate. The nucleotides can be determined using the same electrode and sample, subsequent to the addition of nucleotidase and adenosine deaminase. Typically, K values below 20 show the fish is very fresh and may be eaten raw. Fish with a K value between 20 and 40 must be cooked but those with a K value above 40 are not fit for human consumption.
Critical K values vary amongst species, but can be a reliable indicator applicable to frozen, smoked or fish stored under modified atmospheres. Clearly, a relatively simple probe that accurately and reproducibly determines fish freshness has significant economic importance to the fish industry. In its absence, freshness is determined completely subjectively by inspection.
Although such biosensors are easy to produce, they do suffer from some significant drawbacks. The reaction is dependent on the concentration of molecular oxygen, which precludes its use in oxygendeprived environments such as in vivo. Also, the potential used is sufficient to cause other redox processes to occur, such as ascorbate oxidation/ reduction, which may interfere with the analyses. Much research has been undertaken on the development of substances that can replace oxygen in these reactions. Generally, oxidases are far more specific for the oxidisable reactant than they are for molecular oxygen itself as the oxidant. Many other materials can act as the oxidant. The optimal properties of such materials include fast electron transfer rates, the ability to be easily regenerated by an electrode reaction and retention within the biocatalytic membrane. In addition, they should not react with other molecules, including molecular oxygen that may be present.
Many such oxidants, now called mediators, have been developed. Their redox reactions, which together transfer the electrons from the substrate to the electrode, so producing the electrical signal, are summarised in Figure 4. As they allow reduced working potentials, interference from natural redox materials is reduced. The mediated biosensor reaction consists of three redox processes:
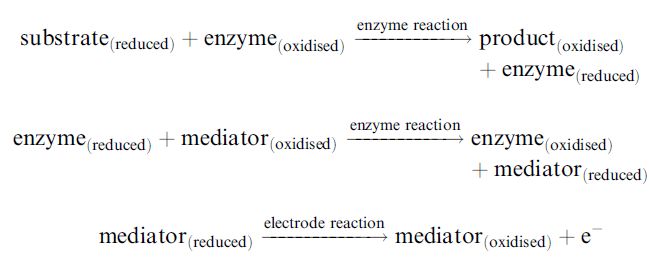
When a steady-state response has been obtained, the rates of all of these processes and the rate of the diffusive flux in must be equal (see Figure 18.4c). Any of these, or a combination, may be the controlling factor. Although the level of response is reproducible, it is therefore difficult to predict its magnitude. Several blood glucose biosensors, for the control of diabetes, have
been built and marketed based on this mediated system (Figure 5).
The sensing area is a single-use disposable electrode, typically produced by screen-printing on to a plastic strip and consisting of an Ag/AgCl reference electrode and a carbon working electrode containing glucose oxidase and a derivatised ferrocene mediator. Both electrodes are covered with wide-mesh hydrophilic gauze to permit even spreading of a blood drop sample and to prevent localised cooling effects due to uneven evaporation. The electrodes are kept dry until use and have a shelf-life of 6 months when sealed in aluminium foil. They can detect glucose concentrations of 2–25mM in single tiny drops of blood (1–4 ml) and accurately display the result within 5–40 s. The pricing is such that the biosensor is sold cheaply with the major profit arising from the sale of the necessary disposable electrodes. Such biosensors are currently used
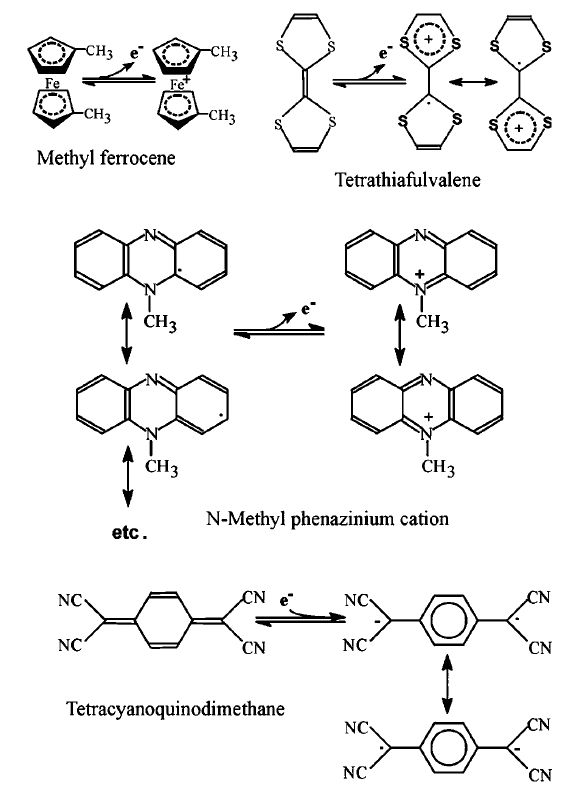
Figure 4. The redox reactions of amperometric biosensor mediators. Tetracyanoquinodimethane acts as a partial electron acceptor whereas ferrocene, tetrathiafulvalene and N-methylphenazinium can all act as partial electron donors.
by millions of people with diabetes in more than 50 countries worldwide. The profits from this area, although by far the greatest in the biosensor industry, are levelling off as, while the world diabetic population stands at over 200 million and is steadily expanding, diabetic patients use their biosensors less often when their diabetes appears under control.
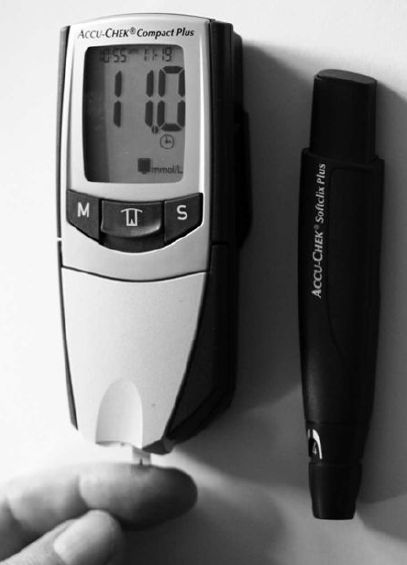
Figure 5. A biosensor for glucose used in diabetes monitoring. A drop of blood is applied to the disposable electrode at the bottom with its glucose concentration being produced on the read-out a few seconds later. On the right is a system for the controlled pricking of a finger.
Although the subject of much research, the use of continuous subcutaneous in vivo glucose sensing, using electrode-based biosensors, has not overcome the difficulties caused by the body’s response and poor patient acceptability. New non-biosensor technologies are being developed, involving near-infrared light which can penetrate tissue and detect glucose in the blood, albeit at low sensitivity and in the face of almost overwhelming interference.
When an oxidase is unable to react rapidly enough with available mediators, horseradish peroxidase, which rapidly reacts with ferrocene mediators, can be included with the enzyme. This catalyses the reduction of the hydrogen peroxide produced by the oxidase and consequent
oxidation of the mediator. In this case, the mediator is acting as an electron donor rather than acceptor. The oxidised mediator then can be rapidly reduced at the electrode at moderate redox potential:
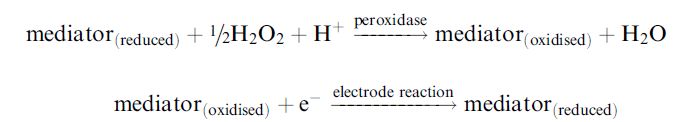
A major advance in the development of microamperometric biosensors came with the discovery that pyrrole can undergo electrochemical oxidative polymerisation (Figure 6) under conditions mild enough to entrap enzymes and mediators at the electrode surface without denaturation. A membrane, entrapping the biocatalyst and mediator, can be formed at the surface of even extremely small electrodes by polymerising pyrrole in the present of biocatalyst. The polypyrrole adheres tightly to platinum, gold or carbon electrodes. This allows silicon chip microfabrication methods to be used and for many different sensors to be laid down on the same chip (Figure 7). Similar membranes may be prepared less wastefully in situ from the oxidation of pyrrole by chloroauric acid to give sensing surfaces with excellent electron transfer properties formed from polypyrrole-immobilised enzymes and gold nanoparticles.
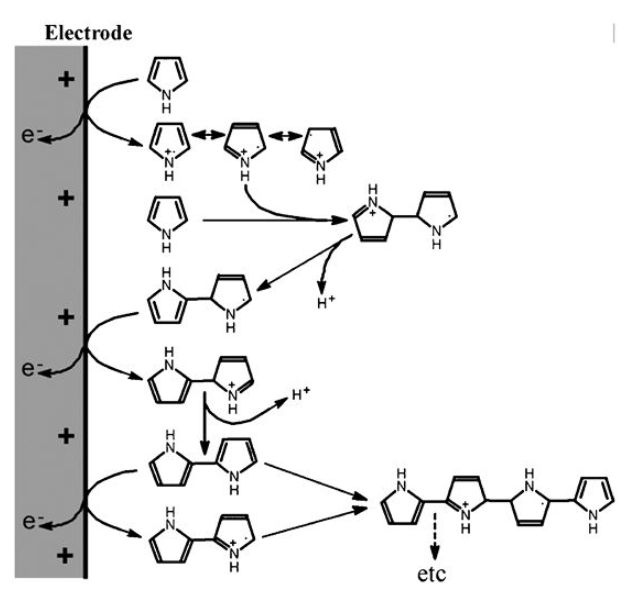
Figure 6. The mechanism for the electrochemical oxidative polymerisation of pyrrole.
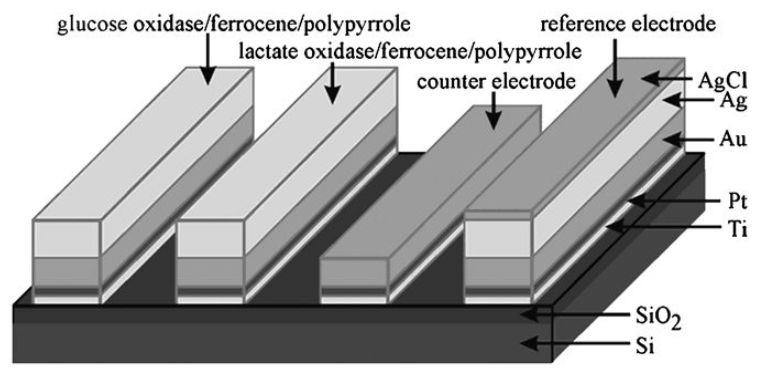
Figure 7. A combined microelectrode for glucose and lactate.
Another advance has been the use of conducting organic salts on the electrode. These allow the direct transfer of electrons from the reduced enzyme to the electrode without the use of any (other) mediator . Conducting organic salts consist of a mixture of two types of planar aromatic molecules, electron donors and electron acceptors , which partially exchange their electrons. These molecules form segregated stacks, containing either the donor or acceptor molecules, with some of the electrons from the donors being transferred to the acceptors. These electrons, which have been partially transferred, are mobile up and down the stacks, giving the organic crystals a high conductivity. There must not be a total electron transfer between the donor and acceptor molecules or the crystal becomes an insulator through lack of electron mobility. These electrodes give the somewhat misleading appearance of direct electron transfer to the electrode. As both the components of the organic salts, in the appropriate redox state, are able to mediate the reaction, it is highly probable that these electrodes are behaving as a highly insoluble mediator prevented from large-scale leakage by electrostatic effects.



|
|
|
|
تفوقت في الاختبار على الجميع.. فاكهة "خارقة" في عالم التغذية
|
|
|
|
|
|
|
أمين عام أوبك: النفط الخام والغاز الطبيعي "هبة من الله"
|
|
|
|
|
|
|
قسم شؤون المعارف ينظم دورة عن آليات عمل الفهارس الفنية للموسوعات والكتب لملاكاته
|
|
|