There are some properties that all liquids have. The liquid that we are most familiar with is probably water, and it has these properties. Other liquids have them as well, which is something to keep in mind.
All liquids have a certain portion of their particles having enough energy to enter the gas phase, and if these particles are at the surface of the liquid, they do so (Figure 10.6 “Evapouration”). The formation of a gas from a liquid at temperatures below the boiling point is called evapouration. At these temperatures, the material in the gas phase is called vapour, rather than gas; the term gas is reserved for when the gas phase is the stable phase.
Figure 10.6 Evapouration

Some particles of a liquid have enough energy to escape the liquid phase to become a vapour.
If the available volume is large enough, eventually all the liquid will become vapour. But if the available volume is not enough, eventually some of the vapour particles will reenter the liquid phase (Figure 1.1 “Equilibrium”). At some point, the number of particles entering the vapour phase will equal the number of particles leaving the vapour phase, so there is no net change in the amount of vapour in the system. We say that the system is at equilibrium. The partial pressure of the vapour at equilibrium is called the vapour pressure of the liquid.
Figure 1.1 Equilibrium

At some point, the number of particles entering the vapour phase will be balanced by the number of particles returning to the liquid. This point is called equilibrium.
Understand that the liquid has not stopped evapourating. The reverse process—condensation—is occurring as fast as evapouration is, so there is no net change in the amount of vapour in the system. The term dynamic equilibrium represents a situation in which a process still occurs, but the opposite process also occurs at the same rate so that there is no net change in the system.
The vapour pressure for a substance is dependent on the temperature of the substance; as the temperature increases, so does the vapour pressure. Figure 10.8 “Plots of Vapor Pressure versus Temperature for Several Liquids” is a plot of vapour pressure versus temperature for several liquids. Having defined vapour pressure, we can also redefine the boiling point of a liquid: the temperature at which the vapour pressure of a liquid equals the surrounding environmental pressure. The normal vapour pressure, then, is the temperature at which the vapour pressure is 760 torr, or exactly 1 atm. Thus boiling points vary with surrounding pressure, a fact that can have large implications on cooking foods at lower- or higher-than-normal elevations. Atmospheric pressure varies significantly with altitude.
Figure 10.8 Plots of Vapor Pressure versus Temperature for Several Liquids
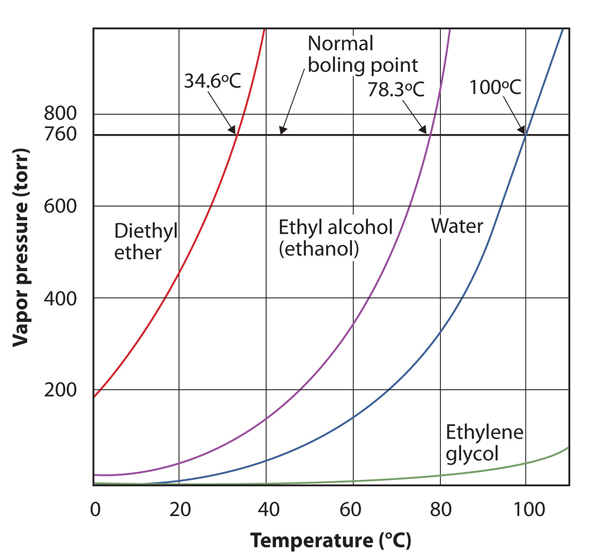
The vapour pressure of a liquid depends on the identity of the liquid and the temperature, as this plot shows.
Example 4
Use Figure 10.8 “Plots of Vapor Pressure versus Temperature for Several Liquids” to estimate the boiling point of water at 500 torr, which is the approximate atmospheric pressure at the top of Mount Everest.
Solution
See the accompanying figure. Five hundred torr is between 400 and 600, so we extend a line from that point on the y-axis across to the curve for water and then drop it down to the x-axis to read the associated temperature. It looks like the point on the water vapour pressure curve corresponds to a temperature of about 90°C, so we conclude that the boiling point of water at 500 torr is 90°C.
Figure 10.9 Using Figure 10.8 “Plots of Vapor Pressure versus Temperature for Several Liquids” to Answer Example 4

By reading the graph properly, you can estimate the boiling point of a liquid at different temperatures.
Test Yourself
Use Figure 10.8 “Plots of Vapor Pressure versus Temperature for Several Liquids” to estimate the boiling point of ethanol at 400 torr.
Answer
about 65°C
The vapour pressure curve for water is not exactly zero at the melting point—0°C. Even ice has a vapour pressure; that is why it sublimes over time. However, the vapour pressures of solids are typically much lower than that of liquids. At −1°C, the vapour pressure of ice is 4.2 torr. At a freezer temperature of 0°F (−17°C), the vapour pressure of ice is only 1.0 torr; so-called deep freezers can get down to −23°C, where the vapour pressure of ice is only 0.6 torr.
















