


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 18-5-2017
Date: 20-7-2019
Date: 1-7-2019
|
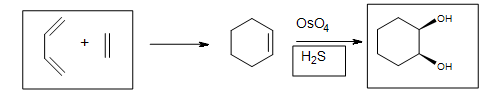
Alkenes are oxidized to cis-1,2-diols by osmium tetroxide (OsO4). The stereospecificity is due to the formation of a cyclic osmate ester intermediate. Osmium tetroxide can be used directly, but it is normally used in catalytic amounts, and is regenerated by N-methylmorpholine-N-oxide.

Examples
Questions:
1. Give the major product.
.bmp?revision=1&size=bestfit&width=131&height=51#fixme)
2. What is the product in the dihydroxylation of (Z)-3-hexene?

3. What is the product in the dihydroxylation of (E)-3-hexene?
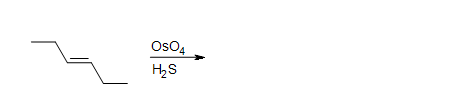
4. Draw the intermediate of this reaction.
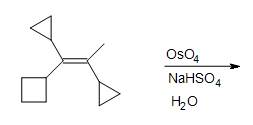
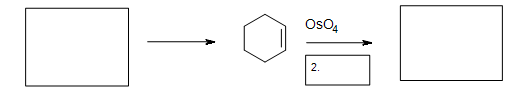
5. Fill in the missing reactants, reagents, and product.
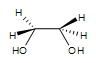
1. A syn-1,2-ethanediol is formed. There is no stereocenter in this particular reaction. The OH groups are on the same side.
2. Meso-3,4-hexanediol is formed. There are 2 stereocenters in this reaction.
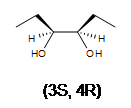
3. A racemic mixture of 3,4-hexanediol is formed. There are 2 stereocenters in both products.
4. A cyclic osmic ester is formed.
5. The Diels-Alder cycloaddition reaction is needed in the first box to form the cyclohexene. The second box needs a reagent to reduce the intermediate cyclic ester. The third box has the product: 1,2-cyclohexanediol.



|
|
|
|
دراسة يابانية لتقليل مخاطر أمراض المواليد منخفضي الوزن
|
|
|
|
|
|
|
اكتشاف أكبر مرجان في العالم قبالة سواحل جزر سليمان
|
|
|
|
|
|
|
المجمع العلمي ينظّم ندوة حوارية حول مفهوم العولمة الرقمية في بابل
|
|
|