


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 3-8-2019
Date: 18-1-2020
Date: 2-8-2019
|
Terminal alkynes are much more acidic than most other hydrocarbons. Removal of the proton leads to the formation of an acetylide anion, RC=C:-. The origin of the enhanced acidity can be attributed to the stability of the acetylide anion, which has the unpaired electrons in an sp hybridized orbital. The stability results from occupying an orbital with a high degree of s-orbital character. There is a strong correlation between s-character in the orbital containing the non-bonding electrons in the anion and the acidity of hydrocarbons. The enhanced acidity with greater s-character occurs despite the fact that the homolytic C-H BDE is larger.
| Compound | Conjugate Base | Hybridization | "s Character" | pKa | C-H BDE (kJ/mol) |
| CH3CH3 | CH3CH2- | sp3 | 25% | 50 | 410 |
| CH2CH2 | CH2CH- | sp2 | 33% | 44 | 473 |
| HCCH | HCC- | sp | 50% | 25 | 523 |
Consequently, acetylide anions can be readily formed by deprotonation using a sufficiently strong base. Amide anion (NH2-), in the form of NaNH2 is commonly used for the formation of acetylide anions.
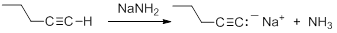
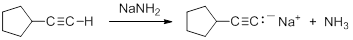



|
|
|
|
تفوقت في الاختبار على الجميع.. فاكهة "خارقة" في عالم التغذية
|
|
|
|
|
|
|
أمين عام أوبك: النفط الخام والغاز الطبيعي "هبة من الله"
|
|
|
|
|
|
|
قسم شؤون المعارف ينظم دورة عن آليات عمل الفهارس الفنية للموسوعات والكتب لملاكاته
|
|
|