


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 18-1-2020
Date: 20-5-2017
Date: 20-1-2020
|
The conformation in which the methyl group is equatorial is more stable, and thus the equilibrium lies in this direction.
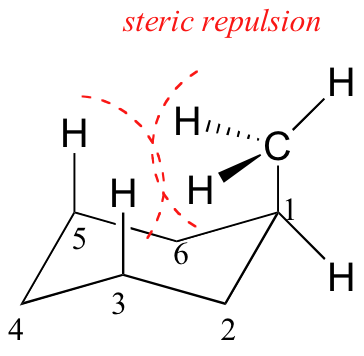
The relative steric hindrance experienced by different substituent groups oriented in an axial versus equatorial location on cyclohexane may be determined by the conformational equilibrium of the compound. The corresponding equilibrium constant is related to the energy difference between the conformers, and collecting such data allows us to evaluate the relative tendency of substituents to exist in an equatorial or axial location. Table 1.1
show some of these free energy values (sometimes referred to as A values).
| Substituent Group | ΔG º (axial–equatorial) | Substituent Group | ΔG º (axial–equatorial) |
|---|---|---|---|
| CH3 | 1.74 kcal mol-1 | F | 0.3 |
| C2H5 | 1.8 | Cl | 0.6 |
| (CH3)2CH | 2.2 | Br | 0.6 |
| (CH3)3C | 4.7 | I | 0.55 |
| CF3 | 2.4 | OH | 0.6 - 1.0 |
| C6H5 | 2.8 | OCH3 | 0.65 |
| CH2=CH | 1.6 | OCOCH3 | 0.75 |
| HC≡C | 0.45 | OSI(CH3)3 | 0.7 |
| CN | 0.2 | SH | 1.2 |
| CHO | 0.7 | NH2 | 1.3 - 1.7 |
| COCH3 | 1.2 | N(CH3)2 | 2.0 |
| CO2H | 1.4 | N3 | 0.5 |
| CO2 Na | 2.0 | NO2 | 1.1 |
| CO2CH3 | 1.3 | Si(CH3)3 | 2.5 |
| COCl | 1.3 | ||
| These "Conformational Energies" or "A values" are -ΔG° 's for axial/equatorial equilibria of substituted cyclohexanes. The data in this table are averaged from several different sources. For a more precise and extensive compilation see: E.L.Eliel and S.H.Wilen, Stereochemistry of Organic Compounds, 1994. | |||
Looking at the energy values in Table 1.1 , it is clear that the apparent "size" of a substituent (in terms of its preference for equatorial over axial orientation) is influenced by its width and bond length to cyclohexane, as evidenced by the fact that an axial vinyl group is less hindered than ethyl, and iodine slightly less than chlorine.
We noted earlier that cycloalkanes having two or more substituents on different ring carbon atoms exist as a pair (sometimes more) of configurational stereoisomers. Now we must examine the way in which favorable ring conformations influence the properties of the configurational isomers. Remember, configurational stereoisomers are stable and do not easily interconvert, whereas, conformational isomers normally interconvert rapidly. In examining possible structures for substituted cyclohexanes, it is useful to follow two principles:



|
|
|
|
تفوقت في الاختبار على الجميع.. فاكهة "خارقة" في عالم التغذية
|
|
|
|
|
|
|
أمين عام أوبك: النفط الخام والغاز الطبيعي "هبة من الله"
|
|
|
|
|
|
|
قسم شؤون المعارف ينظم دورة عن آليات عمل الفهارس الفنية للموسوعات والكتب لملاكاته
|
|
|