


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 21-1-2022
Date: 28-8-2018
Date: 27-8-2018
|
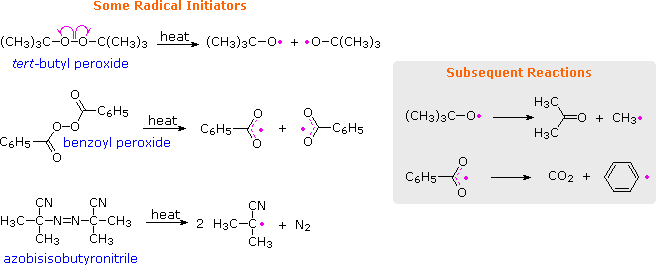
In contrast to stronger C–C and C–H bonds, the very weak O–O bonds of peroxides are cleaved at relatively low temperatures ( 80 to 150 ºC ), as shown in the following equations. The resulting oxy radicals may then initiate other reactions, or may decompose to carbon radicals, as noted in the shaded box. The most commonly used peroxide initiators are depicted in the first two equations.
Organic azo compounds (R–N=N–R) are also heat sensitive, decomposing to alkyl radicals and nitrogen. Azobisisobutyronitrile (AIBN) is the most widely used radical initiator of this kind, decomposing slightly faster than benzoyl peroxide at 70 to 80 ºC. The thermodynamic stability of nitrogen provides an overall driving force for this decomposition, but its favorable rate undoubtedly reflects weaker than normal C-N bonds.



|
|
|
|
الصين.. طريقة لمنع تطور قصر النظر لدى تلاميذ المدارس
|
|
|
|
|
|
|
ماذا سيحدث خلال كسوف الشمس يوم السبت؟
|
|
|
|
|
|
|
قسم الشؤون الدينية يختتم محاضراته الرمضانية في صحن مرقد أبي الفضل العباس (عليه السلام)
|
|
|