


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 27-9-2019
Date: 28-5-2017
Date: 28-8-2019
|
The last example of reversible addition is that of hydrogen cyanide (HC≡N), which adds to aldehydes and many ketone to give products called cyanohydrins.
RCH=O + H–C≡N  RCH(OH)CN (a cyanohydrin)
RCH(OH)CN (a cyanohydrin)
Since hydrogen cyanide itself is an acid (pKa = 9.25), the addition is not acid-catalyzed. In fact, for best results cyanide anion, C≡N(-) must be present, which means that catalytic base must be added. Cyanohydrin formation is weakly exothermic, and is favored for aldehydes, and unhindered cyclic and methyl ketones. Two examples of such reactions are shown below.
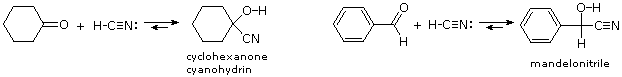
The cyanohydrin from benzaldehyde is named mandelonitrile. The reversibility of cyanohydrin formation is put to use by the millipede Apheloria corrugata in a remarkable defense mechanism. This arthropod releases mandelonitrile from an inner storage gland into an outer chamber, where it is enzymatically broken down into benzaldehyde and hydrogen cyanide before being sprayed at an enemy.



|
|
|
|
4 أسباب تجعلك تضيف الزنجبيل إلى طعامك.. تعرف عليها
|
|
|
|
|
|
|
أكبر محطة للطاقة الكهرومائية في بريطانيا تستعد للانطلاق
|
|
|
|
|
|
|
العتبة العباسية المقدسة تبحث مع العتبة الحسينية المقدسة التنسيق المشترك لإقامة حفل تخرج طلبة الجامعات
|
|
|