


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 19-12-2015
Date: 10-5-2020
Date: 27-3-2017
|
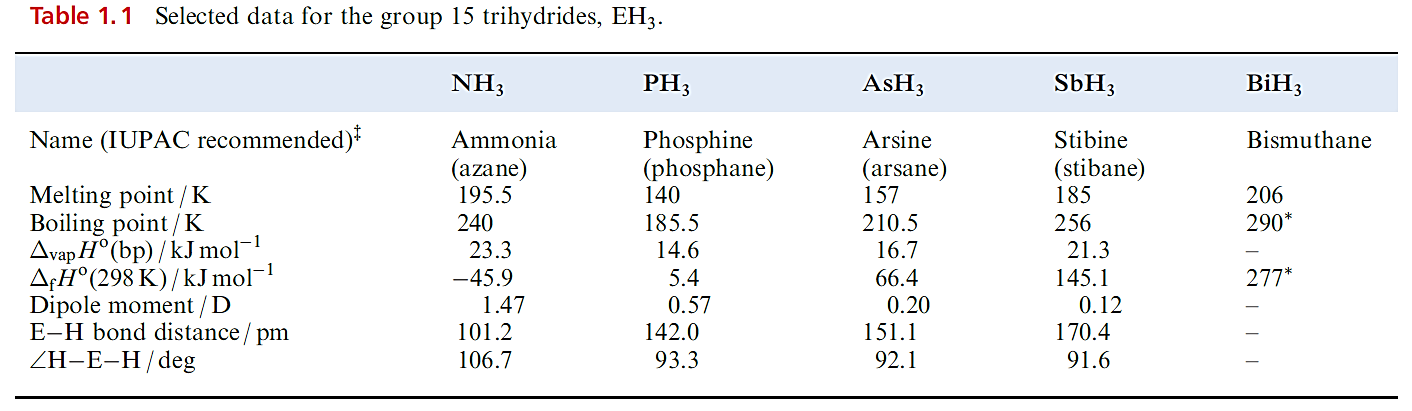
Trihydrides, EH3 (E=N, P, As, Sb and Bi)
Each group 15 element forms a trihydride, selected properties of which are given in Table 1.1; the lack of data for BiH3 stems from its instability. The variation in boiling points ( Table 1.1) is one of the strongest pieces of evidence for hydrogen bond formation by nitrogen. Further evidence comes from the fact that NH3 has a greater value of ΔvapHo and surface tension than the later trihydrides. Thermal stabilities of these compounds decrease down the group (BiH3 decomposes above 228 K), and this trend is reflected in the bond enthalpy terms. Ammonia is the only trihydride to possess a negative value of ΔfHo (Table 1.1).
Ammonia is obtained by the action of H2O on the nitrides of Li or Mg by heating [NH4] salts with base or by reducing a nitrate or nitrite in alkaline solution with Zn or Al.
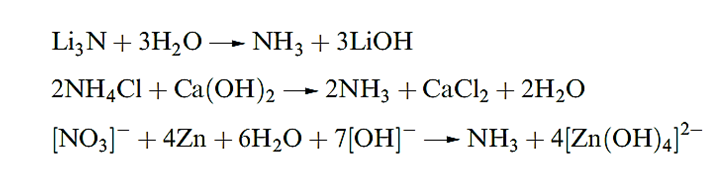
Trihydrides of the later elements are best made by method as in below or by acid hydrolysis of phosphides, arsenides, antimonides or bismuthides. Phosphine can also be made as below, [PH4]I being prepared from P2I4.
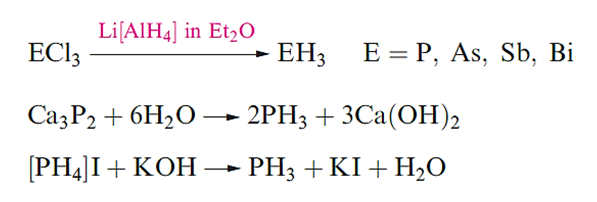
The industrial manufacture of NH3 involves the Haber process (reaction below), and the manufacture of the H2 required contributes significantly to the overall cost of the process.
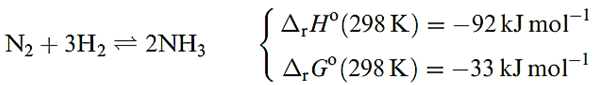
The Haber process is a classic application of physicochemical principles to a system in equilibrium. The decrease in number of moles of gas means that ΔrSo(298 K) is negative. For industrial viability, NH3 must be formed in optimum yield and at a reasonable rate; increasing the temperature increases the rate of reaction, but decreases the yield since the forward reaction is exothermic. At a given temperature, both the equilibrium yield and the reaction rate are increased by working at high pressures; the presence of a suitable catalyst also increases the rate; the rate determining step is the dissociation of N2 into N atoms m chemisorbed onto the catalyst. The optimum reaction conditions are T = 723 K, P = 202 600 kPa, and Fe3O4 mixed with K2O, SiO2 and Al2O3 as the heterogeneous catalyst; the Fe3O4 is reduced to give the catalytically active α-Fe. The NH3 formed is either liquefied or dissolved in H2O to form a saturated solution of specific gravity 0.880.
Ammonia is a colourless gas with a pungent odour; Table 1.1 lists selected properties and structural data for the trigonal pyramidal molecule the barrier to inversion for which is very low (24 kJ mol-1). Oxidation products of NH3 depend on conditions. Reaction below occurs on combustion in O2, but at ≈1200K in the presence of a Pt/Rh catalyst and a contact time of ≈1 ms, the less exothermic reaction takes place. This reaction forms part of the manufacturing process for HNO3 .
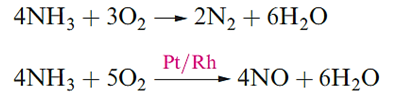
The solubility of NH3 in water is greater than that of any other gas, doubtless because of hydrogen bond formation between NH3 and H2O. The equilibrium constant (at 298 K) shows that nearly all the dissolved NH3 is non-ionized, consistent with the fact that even dilute solutions retain the characteristic smell of NH3. Since Kw = 10-14, it follows that the aqueous solutions of [NH4]+ salts of strong acids (e.g. NH4Cl) are slightly acidic.
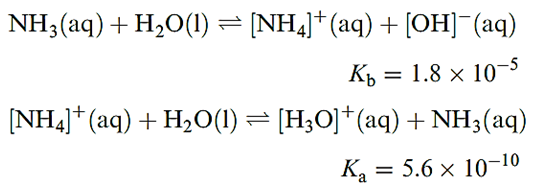
Ammonium salts are easily prepared by neutralization reactions. Industrial syntheses are carried out using the Solvay process both ammonium sulfate and nitrate are important fertilizers, and NH4NO3 is a component of some explosives

Detonation of NH4NO3 may be initiated by another explosion, and ammonium perchlorate is similarly metastable with respect to oxidation of the [NH4]+ cation by the anion; NH4ClO4 is used in solid rocket propellants, e.g. in the booster rockets of the space shuttle. ‘Technical ammonium carbonate’ (used in smelling salts) is actually a mixture of [NH4][HCO3] and [NH4][NH2CO2] (ammonium carbamate); the latter is prepared by passing NH3, CO2 and steam into a lead chamber, and smells strongly of NH3 because carbamic acid is an extremely weak acid (scheme below). Pure carbamic acid (H2NCO2H) has not been isolated; the compound dissociates completely at 332 K.
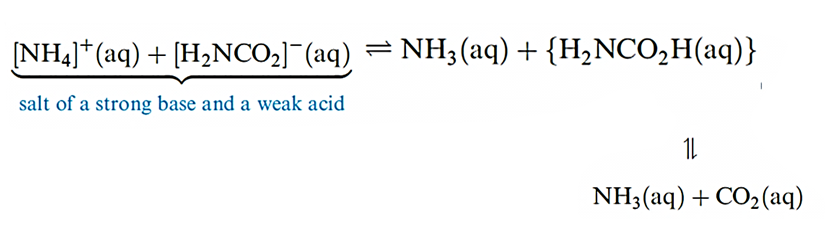
Ammonium salts often crystallize with lattices similar to those of the corresponding K, Rb or Cs salts. The [NH4]+ ion can be approximated to a sphere with rion = 150 pm, a value similar to that of Rb.



|
|
|
|
"عادة ليلية" قد تكون المفتاح للوقاية من الخرف
|
|
|
|
|
|
|
ممتص الصدمات: طريقة عمله وأهميته وأبرز علامات تلفه
|
|
|
|
|
|
|
المجمع العلمي للقرآن الكريم يقيم جلسة حوارية لطلبة جامعة الكوفة
|
|
|