


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 12-1-2017
Date: 11-6-2020
Date: 7-7-2020
|
Silica, silicates and aluminosilicates
Silica, SiO2, is an involatile solid and occurs in many different forms, nearly all of which possess lattice structures constructed of tetrahedral SiO4 building blocks. Each unit is connected to the next by sharing an oxygen atom to give Si-O-Si bridges. At atmospheric pressure, three polymorphs of silica exist; each is stable within a characteristic temperature range, but possesses a low-temperature (a) and hightemperature (b) modification (Figure 1.1).
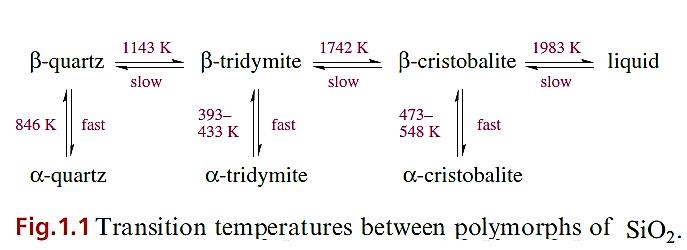
The structure of β-cristobalite and its relationship to that of diamond. The different polymorphs of silica resemble β-cristobalite in having tetrahedral SiO4- units, but each is made unique by exhibiting a different arrangement of these building blocks. α-Quartz has an interlinked helical chain structure and is optically active because the chain has a handedness. It is also piezoelectric and is therefore used in crystal oscillators and filters for frequency control and in electromechanical devices such as microphones and loudspeakers.

A piezoelectric crystal is one that generates an electric field (i.e. develops charges on opposite crystal faces when subjected to mechanical stress) or that undergoes some change to atomic positions when an electric field is applied to it; such crystals must lack a centre of symmetry (e.g. contain tetrahedral arrangements of atoms). Their ability to transform electrical oscillations into mechanical vibration, and vice versa, is the basis of their use in, e.g., crystal oscillators. Transitions from one polymorph to another involve initial Si_O bond cleavage and require higher temperatures than the changes between α- and β-forms of one polymorph. When liquid silica cools, it forms a non-crystalline glass consisting of an infinite lattice assembled from SiO4 tetrahedra connected in a random manner. Only a few oxides form glasses (e.g. B2O3, SiO2, GeO2, P2O5 and As2O5) since the criteria for a random assembly are:
When silica glass is heated to ≈1750 K, it becomes plastic and can be worked in an oxy-hydrogen flame. Silica glass apparatus is highly insensitive to thermal shock owing to the low coefficient of thermal expansion of silica. Borosilicate glass (Pyrex) contains 10–15% B2O3 and has a lower melting point than silica glass. Soda glass contains added alkali which converts some of the Si_O_Si bridges in the silica network into terminal Si=O groups, reducing the melting point below that of borosilicate glass.
In all forms of silica mentioned so far, the Si_Obondlength is ≈160 pm and the Si_O_Si bond angle ≈1448, values close to those in (H3Si)2O.
By heating silica under very high pressure, a rutile form containing 6-coordinate Si is formed in which the Si_O bond length is 179pm (compare with the sum of rcov(Si)=118pm and rcov(O) =73pm). This form of silica is more dense and less reactive than ordinary forms. Silica is not attacked by acids other than HF, with which it forms [SiF6]2-. Fusion of SiO2 with alkali leads to the formation of silicates. Although esters of type Si(OR)4 are known, no well-defined ‘silicic acid’ (H4SiO4) has been established.
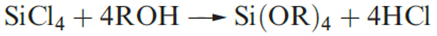
Normal silica is only very slowly attacked by alkali, but silicates are readily formed by fusion of SiO2 and metal hydroxides, oxides or carbonates. The range of known silicates is large and they, and the aluminosilicates (see later), are extremely important, both in nature and for commercial and industrial purposes. Sodium silicates of variable composition are made by heating sand (which is impure quartz containing, e.g., iron(III) oxide) with Na2CO3 at ≈1600K. If the sodium content is high (Na-Si ≈3.2–4.1), the silicates are watersoluble and the resulting alkaline solution (water glass) contains ions such as [SiO(OH)3]- and [SiO2(OH)2]2-; water glass is used commercially in detergents where it controls the pH and degrades fats by hydrolysis. If the Na content is low, the silicate ions consist of large polymeric species and their Na salts are insoluble in water. Equilibrium between the different species is attained rapidly at pH>10, and more slowly in less alkaline solutions. The Earth’s crust is largely composed of silica and silicate minerals, which form the principal constituents of all rocks and of the sands, clays and soils that result from degradation of rocks. Most inorganic building materials are based on silicate minerals and include natural silicates such as sandstone, granite and slate, as well as manufactured materials such as cement, concrete and ordinary glass.
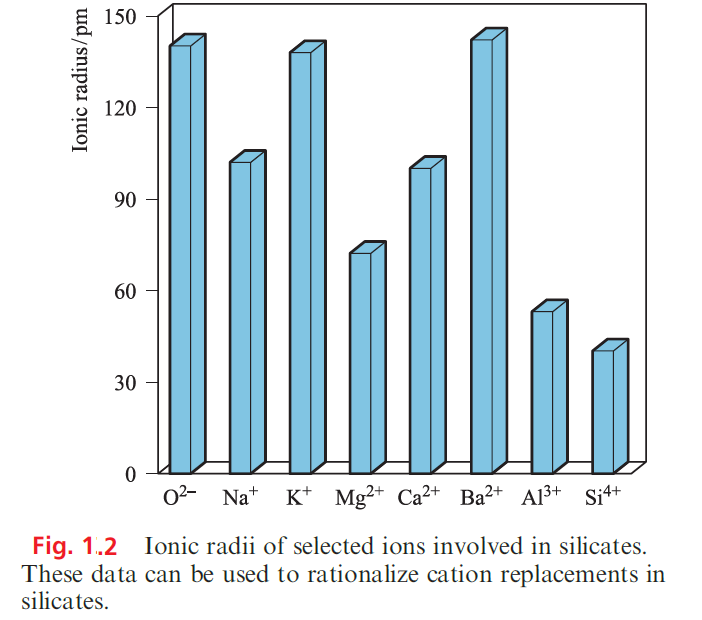
The latter is Bmanufactured by fusing together limestone, sand and Na2CO3. Clays are used in the ceramics industry and mica as an electrical insulator. Fibrous asbestos once had extensive use in heat- and fire-resistant materials, but the health risks associated with the inhalation of asbestos fibres are now well established and alternative heat- and fire-proofing materials are replacing asbestos. We discuss uses of zeolites later in the section. It is universal practice to describe silicates in terms of a purely ionic model. However, although we might write Si4+, the 4+ charge is unlikely on ionization energy grounds and is incompatible with the commonly observed Si_O_Si bond angle of ≈1408. Figure 1.2 compares the ionic radii of ions commonly present in silicates; the value for the ‘Si4+’ ion is an estimate. Since the Al3+ and Si4+ ions are similar sizes, replacement is common and leads to the formation of aluminosilicates. If Al3+ replaces Si4+, however, an extra singly charged cation must be present to maintain electrical neutrality. Thus, in the feldspar orthoclase, KAlSi3O8, the anion [AlSi3O8]- is readily recognized as being related to SiO2 (i.e. [AlSi3O8]- is isoelectronic with Si4O8) and [AlSi3O8]- possesses the structure of quartz with one-quarter of the Si replaced by aluminium; the K ions occupy cavities in the relatively open lattice. Double replacements are also common, e.g. {Na+ Si4+} replaced by {Ca2+ Al3+} (look at the radii comparisons in Figure 1.2).
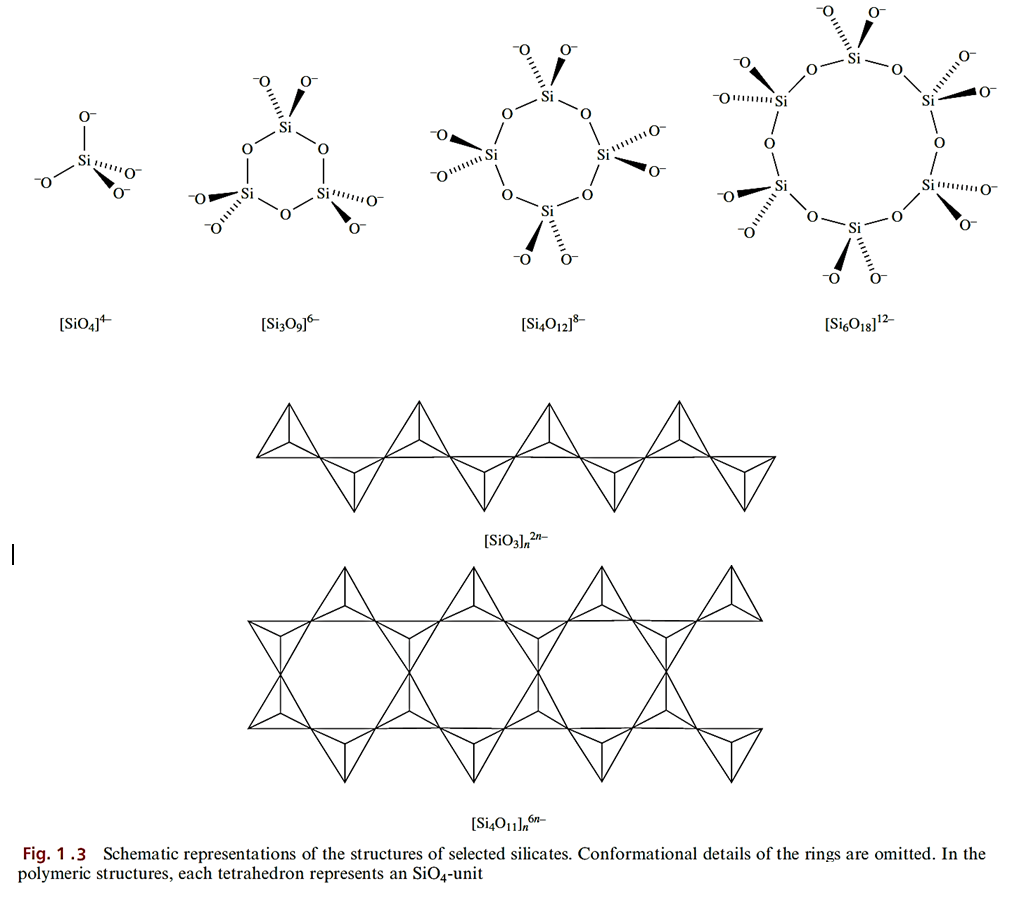
The overwhelming majority of silicates have structures based on SiO4 tetrahedra which, by sharing O atoms, assemble into small groups such as 1.1, cyclic motifs, infinite chains, infinite layers or infinite three-dimensional networks. Sharing an atom only involves corners of tetrahedra; sharing an edge would bring two O2- ions too close together.

Of the metal ions most commonly occurring in silicates, the coordination numbers with respect to O2- ions are: 4 for Be2+, 4 or 6 for Al3+, 6 for Mg2+, Fe3+ or Ti4+, 6 or 8 for Na+, and 8 for Ca2+.
The simplest silicates contain the [SiO4]4- ion and include Mg2SiO4 (olivine) and the synthetic β-Ca2SiO4 (which is an important constituent of cement, setting to a hard mass when finely ground and mixed with water). The mineral thortveitite, Sc2Si2O7 (a major source of scandium), contains discrete [Si2O7]6- ions. The cyclic ions [Si3O9]6- and [Si6O18]12- occur in Ca3Si3O9 (α-wollastonite) and Be3Al2Si6O18 (beryl) respectively, while [Si4O12]8- is present in the synthetic salt K8Si4O12. Short-chain silicates are not common, although [Si3O10]8_ occurs in a few rare minerals. If the SiO4 tetrahedra sharing two corners form an infinite chain, the Si=O ratio is 1-3 (Figure 1.3).
Cage structures have been observed in some synthetic silicates and two examples are shown in Figure 1.4.
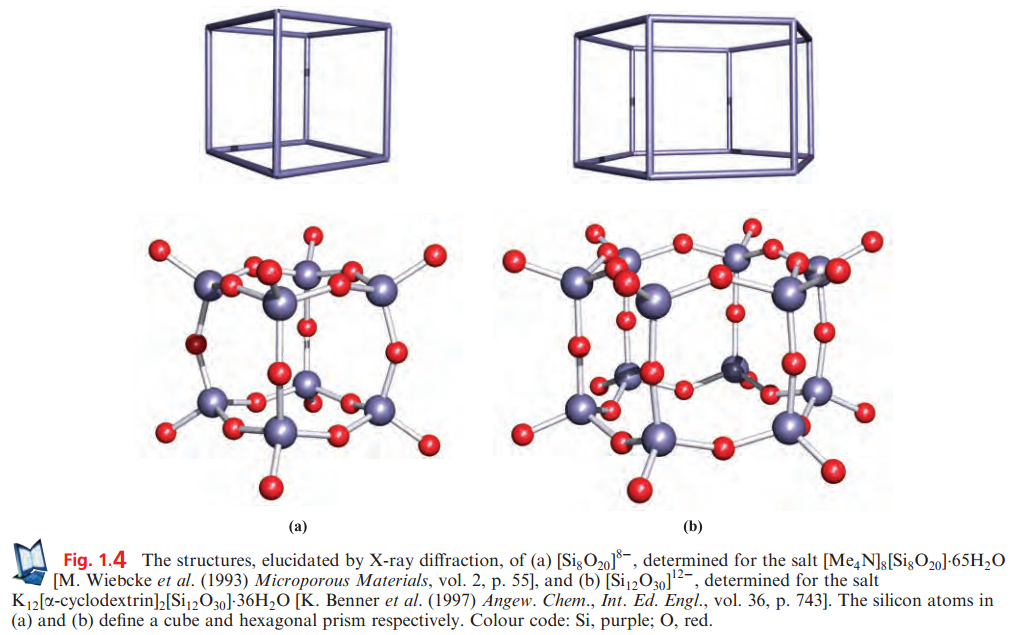
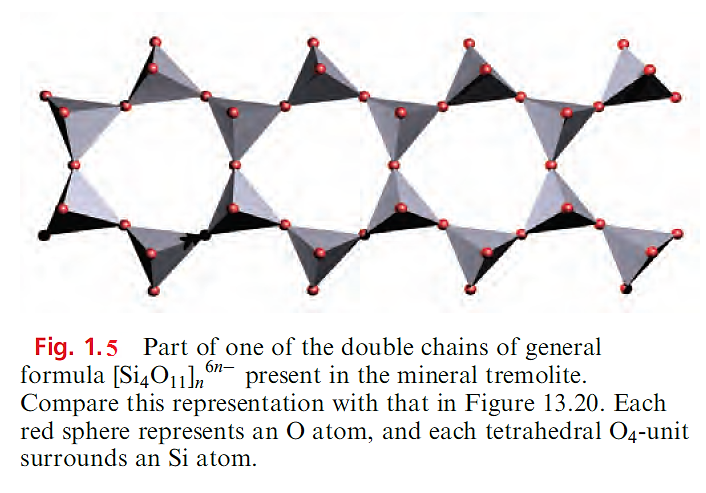
Such chains are present in CaSiO3 (β-wollastonite) and CaMg(SiO3)2 (diopside, a member of the pyroxene group of minerals which possess [SiO3]n2n- chains). Although infinite chains are present in these minerals, the relative orientations of the chains are different. Asbestos consists of a group of fibrous minerals, some of which (e.g. Ca2Mg5(Si4O11)2(OH)2, tremolite) contain the double-chain silicate [Si4O11]n6n- shown in Figures 1.3 and 1.5.
More extended cross-linking of chains produces layer structures of composition [Si2O5]2-; ring sizes within the layers may vary. Such sheets occur in micas and are responsible for the characteristic cleavage of these minerals into thin sheets.
Talc, characterized by its softness, has the composition Mg3(Si2O5)2(OH)2; Mg2+ ions are sandwiched between composite layers each containing [Si2O5]2- sheets and [OH]- ions, and the assembly can be represented by the sequence {Si2O52-}{OH}{Mg2+}3{OH-} {Si2O52- }. This is electrically neutral, allowing talc to cleave readily in a direction parallel to the sandwich. A consequence of this cleavage is that talc is used as a dry lubricant, e.g. in personal care preparations.
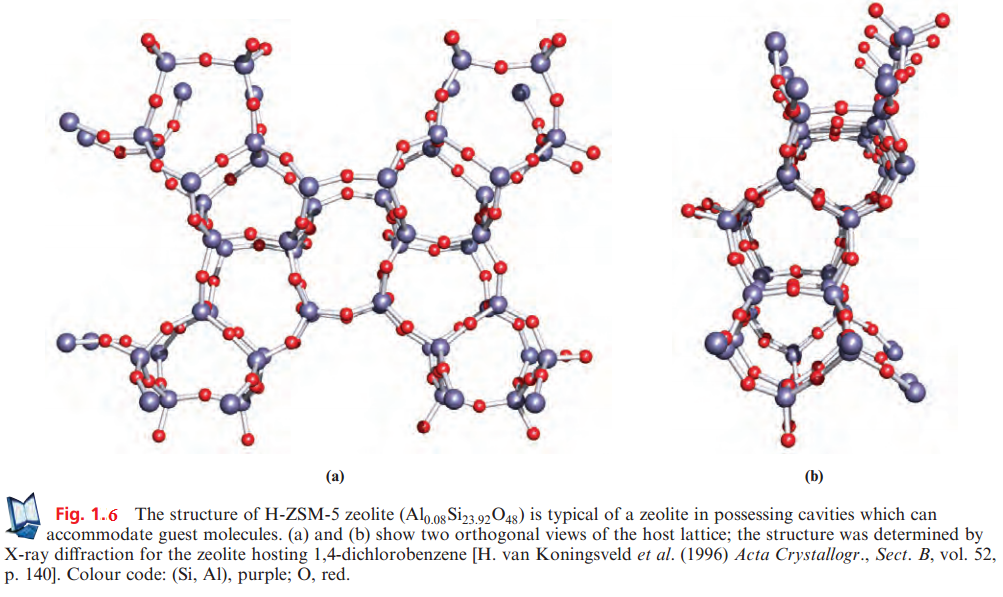
Infinite sharing of all four oxygen atoms of the SiO4 tetrahedra gives a composition SiO2 (see earlier) but partial replacement of Si by Al leads to anions [AlSinO2n+2]-, [Al2SinO2n+2]2- etc. Minerals belonging to this group include orthoclase (KAlSi3O8), albite (NaAlSi3O8), anorthite (CaAl2Si2O8) and celsian (BaAl2Si2O8). Feldspars are aluminosilicate salts of K+, Na+, Ca2+ or Ba2+ and constitute an important class of rock-forming minerals; they include orthoclase, celsian, albite and anorthite. In feldspars, the holes in the structure that accommodate the cations are quite small. In zeolites, the cavities are much larger and can accommodate not only cations but also molecules such as H2O, CO2, MeOH and hydrocarbons. Commercially and industrially, zeolites (both natural and synthetic) are extremely important. The Al : Si ratio varies widely among zeolites; Al-rich systems are hydrophilic and their ability to take up H2O leads to their use as laboratory drying agents (molecular sieves). Different zeolites contain different-sized cavities and channels, permitting a choice of zeolite to effect selective molecular adsorption. Silicon-rich zeolites are hydrophobic. Catalytic uses of zeolites are widespread, e.g. the synthetic zeolite ZSM-5 with composition Nan[AlnSi96-n O192].16H2O (n < 27) catalyses benzene alkylation, xylene isomerization and conversion of methanol to hydrocarbons (for motor fuels). Figure 1.6 illustrates the cavities present in zeolite H-ZSM-5.† Electrical neutrality upon Al-for-Si replacement can also be achieved by converting O- to a terminal OH group.



|
|
|
|
"عادة ليلية" قد تكون المفتاح للوقاية من الخرف
|
|
|
|
|
|
|
ممتص الصدمات: طريقة عمله وأهميته وأبرز علامات تلفه
|
|
|
|
|
|
|
المجمع العلمي للقرآن الكريم يقيم جلسة حوارية لطلبة جامعة الكوفة
|
|
|